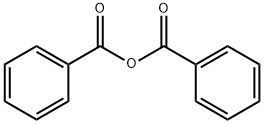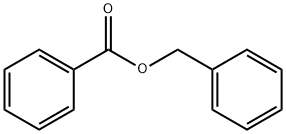
Benzyl benzoate synthesis
- Product Name:Benzyl benzoate
- CAS Number:120-51-4
- Molecular formula:C14H12O2
- Molecular Weight:212.24

100-44-7
658 suppliers
$13.50/250G
65-85-0
1441 suppliers
$10.00/25g

120-51-4
884 suppliers
$6.00/25g
Yield:120-51-4 100%
Reaction Conditions:
with triethylamine at 90; for 2 h;Ionic liquid;Reagent/catalyst;Solvent;
Steps:
Esterification in IL or in Molecular Solvents
The amount of 0.3 g of IL was weighed in a screw-capped 3-ml vial, equipped with a magnetic stirrer; thereafter, the carboxylic acid was added, and then the amine was introduced by a syringe (TEA=benzoic acid1.2:1.0). The mixture was magnetically stirred and heated to 90 C until a clear colorless liquid phase was obtained. Finally, benzyl chloride (BnCl=benzoic acid 1.1:1 molar ratio) was added by means a syringe to the homogenous mixture. At the end of reaction, after cooling to rt, water and ethyl ether were added to the reaction mixture, and the mixture was transferred as quantitatively as possible (rinsing with both water and ethyl ether were carried out) to a separatory funnel, where also t-butylbenzene was added, as internal standard. The organic phase was washed twice with water (10mL), twice with NaHCO3 solution, and twice with 3M HCl, and with water again until neutrality; it was thereafter dried over anhydrous Na2SO4 and analyzed by GLC. For reaction in traditional molecular solvents, the amount of 1 mmol of carboxylic acid was weighed in a screw-capped 5-ml vial, equipped with a magnetic stirrer, and then 3mL of solvent were added. Thereafter, the amine, in the chosen molar ratio, was introduced by a syringe. The homogeneous mixture was magnetically stirred and heated to 90 C. Finally, benzyl chloride (BnCl=carboxylic acid 1.1:1 molar ratio) was added by means a syringe to the homogenous mixture. Further elaboration was as described previously.
References:
Cardellini, Fabio;Brinchi, Lucia;Germani, Raimondo;Tiecco, Matteo [Synthetic Communications,2014,vol. 44,# 22,p. 3248 - 3256]
591-50-4
502 suppliers
$10.00/1g
201230-82-2
1 suppliers
inquiry

100-51-6
1438 suppliers
$5.00/100g

120-51-4
884 suppliers
$6.00/25g
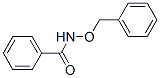
3532-25-0
1 suppliers
inquiry

120-51-4
884 suppliers
$6.00/25g

100-52-7
975 suppliers
$20.37/250g

100-51-6
1438 suppliers
$5.00/100g

120-51-4
884 suppliers
$6.00/25g