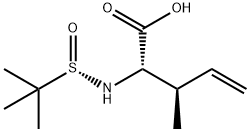
(2S,3R,SS)-2-(tert-butylsulfinamido)-3-methylpent-4-enoic acid synthesis
- Product Name:(2S,3R,SS)-2-(tert-butylsulfinamido)-3-methylpent-4-enoic acid
- CAS Number:1461641-97-3
- Molecular formula:C10H19NO3S
- Molecular Weight:233.33
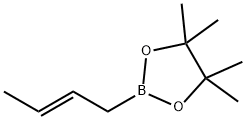
69611-02-5
25 suppliers
$82.00/100mg
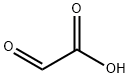
298-12-4
434 suppliers
$5.00/25g
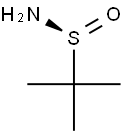
343338-28-3
374 suppliers
$4.00/1g

1461641-97-3
9 suppliers
inquiry
Yield:1461641-97-3 72%
Reaction Conditions:
Stage #1: Glyoxilic acid;(S)-2-methylpropane-2-sulfinamide in dichloromethane;water at 20; for 42 h;Molecular sieve;
Stage #2: (E)-2-(but-2-enyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in dichloromethane;water at 20; for 23 h;diastereoselective reaction;
Steps:
4.2.3 (2S,3R,SS)-2-(tert-Butylsulfinamido)-3-methylpent-4-enoic acid ent-7c
General procedure: A mixture of (R)-tert-butanesulfinamide (R)-4 (121 mg, 1.00 mmol), glyoxylic acid monohydrate 1 (92.0 mg, 1.00 mmol) and powdered molecular sieves 3 ? (648 mg) in CH2Cl2 (9.0 mL) was stirred for 42 h at room temperature. Allylboronic acid pinacol ester 2a (168 mg, 1.00 mol) was added to the mixture, and the resulting mixture was stirred for 23 h at room temperature. The reaction mixture was filtered through a pad of Celite and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (CHCl3/MeOH, 9:1) to afford allylglycine derivative 7a (194 mg, 88%). Other amino acid derivatives 7c, d, f and their enantiomers ent-7c, d, f were also synthesized on a 1.00 mmol scale. For the syntheses of ent-7c, d, and f, (S)-tert-butanesulfinamide (S)-4 was used instead of (R)-tert-butanesulfinamide (R)-4.
References:
Sugiyama, Shigeo;Imai, Saori;Ishii, Keitaro [Tetrahedron Asymmetry,2013,vol. 24,# 18,p. 1069 - 1074]