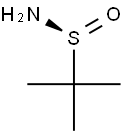
(S)-(-)-2-Methyl-2-propanesulfinamide synthesis
- Product Name:(S)-(-)-2-Methyl-2-propanesulfinamide
- CAS Number:343338-28-3
- Molecular formula:C4H11NOS
- Molecular Weight:121.2
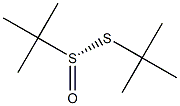
60011-16-7

343338-28-3
The general procedure for the synthesis of S-tert-butylsulfinamide from (S)-tert-butylsulfinic acid thio-tert-butyl ester was as follows: in the third step, 120 mL of diethoxymethane was added to the reaction flask and the reaction temperature was controlled between -60 °C and -70 °C. Liquid ammonia (23.8 g, 1.40 mol) was slowly added dropwise to 2.6 mL of 2.3 M n-hexyl lithium (0.96 mol) solution, at which point a white solid gradually appeared in the solution. The reaction was maintained at this condition for 0.5 hr. Subsequently, a mixture of (S)-tert-butylsulfinyl tert-butyl thioester (169.8 g, 0.87 mol, 96.5% ee) and ethyl chloride (80.4 g, 1.25 mol) was added dropwise to the above mentioned diethoxymethane solution (which is the solution of the product obtained from the second step of the reaction) and cooled to 0 °C. After the dropwise addition was completed, the reaction was continued under stirring for 1 hour to ensure that the reaction was complete. Upon completion of the reaction, the reaction solution was concentrated to dryness under reduced pressure, 1400 mL of methyl tert-butyl ether was added, and the solvent was removed by filtration through diatomaceous earth and subsequent evaporation. The residue was slurried with 135 mL of n-heptane between -10 °C and 0 °C to afford fine needle-like crystals of S-tert-butylsulfinamide 79.1 g in 75% yield, 99.7% HPLC purity, and an enantiomeric excess value (ee) of 99.4%.

60011-16-7
72 suppliers
$41.00/250mg

343338-28-3
394 suppliers
$4.00/1g
Yield:343338-28-3 75%
Reaction Conditions:
with ammonia;n-hexyllithium at -60 - 0; for 1 h;
Steps:
2.3 Example 2
In the third step, 120 mL of diethoxymethane was added to the reaction flask, and the temperature was controlled between -60 °C and -70 °C. Liquid ammonia (23.8 g, 1.40 mol) was added dropwise to 2.6 mL of 2.3M n-hexyllithium (0.96). Molar), the solution appeared during the process of white solids, incubation reaction 0.5 hours. Then (S)-tert-butylsulfinyl tert-butyl thioester (169.8 g, 0.87 mol, 96.5% ee) and ethyl chloride (80.4 g, 1.25 mol) were added dropwise to the diethoxymethane solution (by The product solution obtained in the second step was cooled to 0° C. and mixed with ethyl chloride). After the dropwise addition was completed, the reaction was incubated for 1 hour while the reaction was stirred and the reaction was completed. After the reaction solution was evaporated to dryness under reduced pressure, 1400 mL of methyl tert-butyl ether was added, filtered through celite, and the solvent was evaporated. 135 mL of n-heptane was slurried between -10°C and 0°C to obtain fine needle crystals. (3)-tert-butyl Sulfonamide 79.1 g, yield 75%, HPLC: 99.7%, 99.4% ee.
References:
CN106478471,2017,A Location in patent:Paragraph 0018; 0019
110-06-5
180 suppliers
$11.00/25g

343338-28-3
394 suppliers
$4.00/1g
![2-Propanesulfinic acid, 2-methyl-, (1R,2S)-2,3-dihydro-1-[[(2,4,6-trimethylphenyl)sulfonyl]amino]-1H-inden-2-yl ester, [S(S)]-](/CAS/20210305/GIF/861821-86-5.gif)
861821-86-5
0 suppliers
inquiry

343338-28-3
394 suppliers
$4.00/1g
![2-Propanesulfinic acid, 2-methyl-, (1R,2S)-2,3-dihydro-1-[[(2,4,6-trimethylphenyl)sulfonyl]amino]-1H-inden-2-yl ester, [S(R)]-](/CAS/20210305/GIF/446021-65-4.gif)
446021-65-4
0 suppliers
inquiry

343338-28-3
394 suppliers
$4.00/1g
![Benzenesulfonamide, N-[(1S,2R)-2-[[(S)-(1,1-dimethylethyl)sulfinyl]oxy]-1-methyl-2-phenylethyl]-4-methyl-](/CAS/20210305/GIF/594836-47-2.gif)
![Benzenesulfonamide, N-[(1S,2R)-2-hydroxy-1-methyl-2-phenylethyl]-4-methyl-](/CAS/20210111/GIF/108591-33-9.gif)