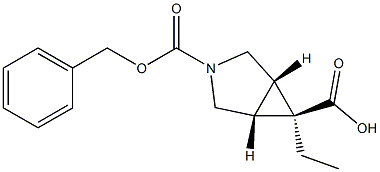
(1R,5S,6r)-3-benzyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate synthesis
- Product Name:(1R,5S,6r)-3-benzyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate
- CAS Number:146726-10-5
- Molecular formula:C16H19NO4
- Molecular Weight:289.33
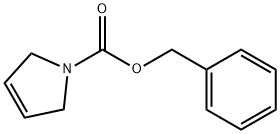
31970-04-4
172 suppliers
$14.14/250mgs:
![(1R,5S,6s)-3-benzyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20150408/GIF/134575-38-5.gif)
134575-38-5
1 suppliers
inquiry
![(1R,5S,6r)-3-benzyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20150408/GIF/146726-10-5.gif)
146726-10-5
4 suppliers
inquiry
Yield:146726-10-5 28% ,134575-38-5 57%
Reaction Conditions:
rhodium(II) acetate in hexane;dichloromethane;ethyl acetate;
Steps:
L [1α,5α,6β]-3-Benzyloxycarbonyl-3-azabicyclo[3.1.0]hexane-6-carboxylic acid, ethyl ester
[1α,5α,6β]-3-Benzyloxycarbonyl-3-azabicyclo[3.1.0]hexane-6-carboxylic acid, ethyl ester A solution of ethyl diazoacatate (5.8 ml, 55 mmol) in methylene chloride (32 ml) was added slowly (over 70 hours, using a syringe pump) to a mixture of 1-benzyloxycarbonyl-3-pyrroline (9.25 g, 50.0 mmol), and rhodium acetate (1.0 g, 2.3 mmol) in methylene chloride (140 ml). At the end of the addition, the reaction mixture was filtered through Celite and concentrated in vacuo . The residue was purified by column chromatography (eluant: 10% ethyl acetate in hexane) to provide recovered starting material (3.2 g, 17.3 mmol) and the title products: [1α,5α,6α]-3-Benzyloxycarbonyl-3-azabicyclo[3.1.0] hexane-6-carboxylic acid, ethyl ester: (2.61 g, 9.02 mmol, 28% yield based on recovered starting material): 1H NMR (CDCl3): 7.32 (m, 5H), 5.08 (s, 2H), 4.10 (q, J=7.4 Hz, 2H), 3.71 (dd, J=14, 11.4 Hz, 2H), 3.49 (m, 2H), 2.07 (m, 2H), 1. (m, 1H), 1.23 (t, J=7.4 Hz, 3H). [1α,5α,6β]-3-Benzyloxycarbonyl-3-azabicyclo[3.1.0] hexane-6-carboxylic acid, ethyl ester: (5.4 g, 18.7 mmol, 57% yield based on recovered starting material): 1H NMR (CDCl3): 7.30 (m, 5H), 5.06 (s, 2H), 3.97 (q, J=7 Hz, 2H), 3.80 (d, J=11.2 Hz, 2H), 3.49 (m, 2H), 1.87 (m, 2H), 1.75 (m, 1H), 1.12 (t, J=7 Hz, 3H).
References:
EP413455,1991,A2
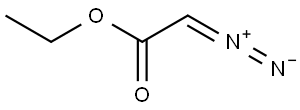
623-73-4
152 suppliers
$27.30/25ML

31970-04-4
172 suppliers
$14.14/250mgs:
![(1R,5S,6s)-3-benzyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20150408/GIF/134575-38-5.gif)
134575-38-5
1 suppliers
inquiry
![(1R,5S,6r)-3-benzyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20150408/GIF/146726-10-5.gif)
146726-10-5
4 suppliers
inquiry
![Ethyl 3-azabicyclo[3.1.0]hexane-6-carboxylate](/CAS/GIF/174456-77-0.gif)
174456-77-0
29 suppliers
$502.75/5MG

501-53-1
407 suppliers
$10.00/1g
![(1R,5S,6r)-3-benzyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20150408/GIF/146726-10-5.gif)
146726-10-5
4 suppliers
inquiry
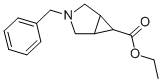
134575-06-7
52 suppliers
$15.00/250mg
![(1R,5S,6r)-3-benzyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20150408/GIF/146726-10-5.gif)
146726-10-5
4 suppliers
inquiry

501-53-1
407 suppliers
$10.00/1g
![(1R,5S,6r)-3-benzyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20150408/GIF/146726-10-5.gif)
146726-10-5
4 suppliers
inquiry