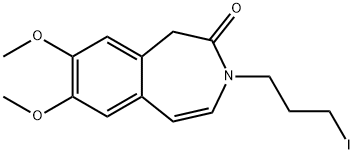
7,8-Dimethoxy-3-(3-iodopropyl)-1,3-dihydro-2H-3-benzazepin-2-one synthesis
- Product Name:7,8-Dimethoxy-3-(3-iodopropyl)-1,3-dihydro-2H-3-benzazepin-2-one
- CAS Number:148870-57-9
- Molecular formula:C15H18INO3
- Molecular Weight:387.21
![(Z)-3-(3-chloropropyl)-7,8-diethyl-1H-benzo[d] azepin-2 (3H)-one](/CAS/GIF/85175-59-3.gif)
85175-59-3

148870-57-9
A mixed solution of 7,8-dimethoxy-3-(3-chloropropyl)-1,3-dihydro-2H-3-benzazepin-2-one (50.00 g, 169 mmol) with sodium iodide (32.75 g, 218 mmol) in methyl isobutyl ketone (375 mL) was reacted under argon protection at 117-118 °C with stirring. The reaction process was monitored by high performance liquid chromatography (HPLC). Upon completion of the reaction, the mixture was concentrated under reduced pressure and the residue was diluted with dichloromethane (375 mL) and water (190 mL). The organic phase was separated, washed with water (190 mL) and concentrated again under reduced pressure. The resulting residue was treated with methyl tert-butyl ether (190 mL) and the resulting suspension was cooled to 0 °C. After continuous stirring at 0 °C for 1 h, the solid product was collected by filtration, washed with methyl isobutyl ether (40 mL) and dried at 23 °C/100 mbar for 2 h. The final product was obtained as 60.10 g of light yellow solid product in 92% yield.
![(Z)-3-(3-chloropropyl)-7,8-diethyl-1H-benzo[d] azepin-2 (3H)-one](/CAS/GIF/85175-59-3.gif)
85175-59-3
234 suppliers
$6.00/250mg

148870-57-9
162 suppliers
$8.00/250mg
Yield:148870-57-9 92%
Reaction Conditions:
with sodium iodide at 117 - 118;Inert atmosphere;
Steps:
Preparation of the compound of formula (III)
A mixture of the chloride of formula (Ilia) (50.00 g; 169 mmol) and sodium iodide (32.75 g; 218 mmol) in methyl isobutyl ketone (375 ml) was heated to 117 - 118°C with stirring under an argon atmosphere. Reaction progress was monitored by HPLC. After completion, the reaction mixture was concentrated in vacuo and the residue was diluted with dichloro methane (375 ml) and water (190 ml). The organic layer was separated, washed with water (190 ml) and concentrated in vacuo. The residue was treated with methyl t- butyl ether (190 ml) and the resulting suspension was cooled to 0°C. After 1 hour of stirring at 0°C, the suspension was filtered, washed with methyl i-butyl ether (40 ml) and dried for two hour at 23°C/100 mbar to afford 60.10 g of yellowish solid (92% yield).
References:
WO2014/114341,2014,A1 Location in patent:Page/Page column 15; 16
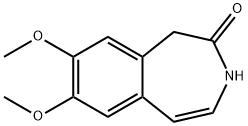
73942-87-7
309 suppliers
$23.00/1g

148870-57-9
162 suppliers
$8.00/250mg
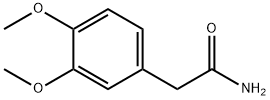
5663-56-9
53 suppliers
$60.00/50mg

148870-57-9
162 suppliers
$8.00/250mg
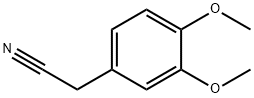
93-17-4
464 suppliers
$7.00/10g

148870-57-9
162 suppliers
$8.00/250mg
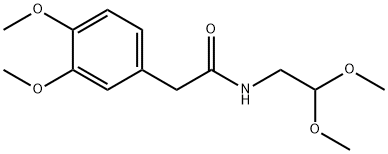
73954-34-4
60 suppliers
inquiry

148870-57-9
162 suppliers
$8.00/250mg