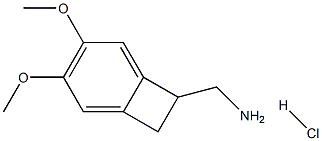
(3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanamine hydrochloride synthesis
- Product Name:(3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanamine hydrochloride
- CAS Number:35202-55-2
- Molecular formula:C11H16ClNO2
- Molecular Weight:229.7032
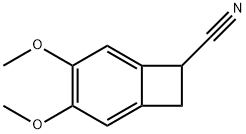
35202-54-1
![(3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanamine hydrochloride](/CAS/20180703/GIF/35202-55-2.gif)
35202-55-2
General procedure for the synthesis of (3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-dien-7-yl)methanamine hydrochloride from 4,5-dimethoxy-1-cyanobenzocyclobutane: reference to EP 0 534 859 Step 1: Preparation of 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-amine hydrochloride. To a solution of 25 g of 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile dissolved in 250 ml of tetrahydrofuran (THF) at room temperature was added slowly and dropwise 312 ml of borane-THF complex solution with continuous stirring for 12 hours. Subsequently, 200 mL of ethanol was added and stirring was continued for 1 hour. 100 ml of a 3.3 N solution of ethyl ether hydrochloride was added slowly and dropwise. Eventually 27.7 g of the target product was obtained in 90% yield with a melting point of 205°C.

35202-54-1
271 suppliers
$9.00/250mg
![(3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanamine hydrochloride](/CAS/20180703/GIF/35202-55-2.gif)
35202-55-2
41 suppliers
$89.00/100mg
Yield:35202-55-2 90%
Reaction Conditions:
Stage #1:3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile with borane-THF in tetrahydrofuran at 20; for 12 h;
Stage #2: with ethanol in tetrahydrofuran for 1 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;diethyl ether
Steps:
3.1 Step 1: 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-amine hydrochloride
Based on EP 0 534 859 Step 1: 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-amine hydrochloride [0053] 312 mL of a molar solution of borane complexed with THF are added dropwise, and whilst stirring at ambient temperature, to a solution of 25 g of 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile in 250 mL of THF and left in contact for 12 hours; 200 mL of ethanol are then added and stirring is carried out for 1 hour. 100 mL of 3.3N ethereal HCl are added dropwise. 27.7 g of the expected product are obtained. [0054] Yield=90% [0055] m.p.=205° C.
References:
LES LABORATOIRES SERVIER;CARRANZA, Maria Del Pilar;GARCIA ARANDA, Maria Isabel;GONZALEZ, José Lorenzo;SANCHEZ, Frédéric US2014/107334, 2014, A1 Location in patent:Paragraph 0052-0055
35249-62-8
64 suppliers
inquiry
![(3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanamine hydrochloride](/CAS/20180703/GIF/35202-55-2.gif)
35202-55-2
41 suppliers
$89.00/100mg
37629-85-9
3 suppliers
inquiry
![(3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanamine hydrochloride](/CAS/20180703/GIF/35202-55-2.gif)
35202-55-2
41 suppliers
$89.00/100mg
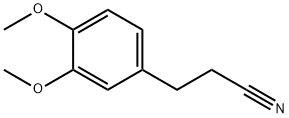
49621-56-9
11 suppliers
inquiry
![(3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanamine hydrochloride](/CAS/20180703/GIF/35202-55-2.gif)
35202-55-2
41 suppliers
$89.00/100mg
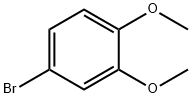
2859-78-1
329 suppliers
$6.00/5g
![(3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methanamine hydrochloride](/CAS/20180703/GIF/35202-55-2.gif)
35202-55-2
41 suppliers
$89.00/100mg