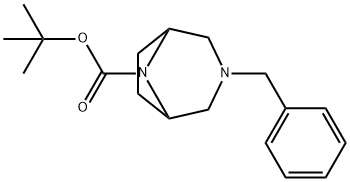
TERT-BUTYL 3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE
- CAS Number:149771-43-7
- Molecular formula:C18H26N2O2
- Molecular Weight:302.41

24424-99-5
![3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE](/CAS/GIF/67571-90-8.gif)
67571-90-8
![TERT-BUTYL 3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE](/CAS/GIF/149771-43-7.gif)
149771-43-7
3-Benzyl-3,8-diazabicyclo[3.2.1]octane (13.5 g, 66.7 mmol) was used as starting material and mixed with triethylamine (6.75 g, 66.7 mmol) and di-tert-butyl dicarbonate (14.56 g, 66.7 mmol) in dichloromethane (100 ml). The reaction mixture was stirred under cooling in an ice bath for 3 hours. After completion of the reaction, the crude product was washed with water (150 ml) and the aqueous phase was then extracted with dichloromethane (50 ml). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give tert-butyl 3-benzyl-3,8-diazabicyclo[3.2.1]octane-8-carboxylate (13 g, 65% yield). The melting point of the product was 58-60°C.

100-39-0
434 suppliers
$10.00/10 g
![8-BOC-3,8-DIAZA-BICYCLO[3.2.1]OCTANE](/CAS/GIF/149771-44-8.gif)
149771-44-8
217 suppliers
$14.00/250mg
![TERT-BUTYL 3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE](/CAS/GIF/149771-43-7.gif)
149771-43-7
42 suppliers
inquiry
Yield:149771-43-7 90%
Reaction Conditions:
with triethylamine in acetonitrile at 50; for 5 h;Inert atmosphere;
Steps:
5.1 Step 1) Synthesis of 3-benzyl-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid tert-butyl ester
3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid tert-butyl ester (0.5 g, 2.4 mmol),Benzyl bromide (0.31 mL, 2.6 mmol) and triethylamine (0.5 ml, 3.6 mmol) were sequentially added to a 100 mL single-mouth bottle.Dissolved in acetonitrile (20 mL),The temperature was gradually raised to 50 ° C for 5 hours under nitrogen protection.The reaction solution was cooled to room temperature and poured into tap water.Ethyl acetate extraction (60 mL × 3), and the organic phase was reused with tap water.The mixture was washed with brine and dried over anhydrous sodium sulfate.Filtered and evaporated to dryness to give a crude product.Purified by column chromatography (petroleum ether / ethyl acetate (v / v) = 40/1-20/1)The title compound was obtained as a white solid(0.68g, 90%).
References:
Guangdong Dongyangguang Pharmaceutical Co., Ltd.;Jin Chuanfei;Xu Tengfei;Liang Haiping CN108727393, 2018, A Location in patent:Paragraph 0228; 0230; 0231

24424-99-5
861 suppliers
$13.50/25G
![3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE](/CAS/GIF/67571-90-8.gif)
67571-90-8
72 suppliers
$125.00/50mg
![TERT-BUTYL 3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE](/CAS/GIF/149771-43-7.gif)
149771-43-7
42 suppliers
inquiry

100-52-7
977 suppliers
$17.30/2g
![8-BOC-3,8-DIAZA-BICYCLO[3.2.1]OCTANE](/CAS/GIF/149771-44-8.gif)
149771-44-8
217 suppliers
$14.00/250mg
![TERT-BUTYL 3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE](/CAS/GIF/149771-43-7.gif)
149771-43-7
42 suppliers
inquiry

24424-99-5
861 suppliers
$13.50/25G

144-55-8
1406 suppliers
$10.00/25g
![3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE](/CAS/GIF/67571-90-8.gif)
67571-90-8
72 suppliers
$125.00/50mg
![TERT-BUTYL 3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE](/CAS/GIF/149771-43-7.gif)
149771-43-7
42 suppliers
inquiry
54221-37-3
38 suppliers
$77.00/5g
![TERT-BUTYL 3-BENZYL-3,8-DIAZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE](/CAS/GIF/149771-43-7.gif)
149771-43-7
42 suppliers
inquiry