
1-[4-(Hydroxymethyl)piperidin-1-yl]-2,2-dimethylpropan-1-one synthesis
- Product Name:1-[4-(Hydroxymethyl)piperidin-1-yl]-2,2-dimethylpropan-1-one
- CAS Number:155535-54-9
- Molecular formula:C11H21NO2
- Molecular Weight:199.29

768290-38-6
4 suppliers
inquiry
![1-[4-(Hydroxymethyl)piperidin-1-yl]-2,2-dimethylpropan-1-one](/CAS/GIF/155535-54-9.gif)
155535-54-9
9 suppliers
inquiry
Yield:155535-54-9 93.4%
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran;ethanol at 0 - 20;
Steps:
A Intermediate 1A
Intermediate IB (1153 g, 4.790 mol) was taken in a mixture of ethanol and dry THF (12 L, 1 : 1) and cooled to 0 °C. Sodium borohydride (542.5 g, 14.35 mol) was added portionwise at 0 °C and then the reaction mixture was allowed to attain rt by stirring overnight. After the completion of reaction, water was added and ethanol and THF were concentrated under reduced pressure. The concentrated mass was dissolved in ethyl acetate and washed with water, brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give Intermediate 1 A (890 g, yield: 93.4%). GCMS: 199 (M+); lH NMR (300 MHz, CDC13) δ 1.28 (s, 9 H, 3 x CH3 of pivaloyl), 1.76 (m, 4 H), 1.80 (m, 1 H), 2.05 (s, 1 H, OH), 2.74 - 2.82 (t, 2 H), 3.50 (d, 2 H), 4.43 - 4.47 (d, 2 H, CH2OH).
References:
WO2014/22349,2014,A1 Location in patent:Paragraph 00144
6457-49-4
356 suppliers
$18.00/25g
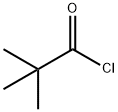
3282-30-2
380 suppliers
$19.85/100ml
![1-[4-(Hydroxymethyl)piperidin-1-yl]-2,2-dimethylpropan-1-one](/CAS/GIF/155535-54-9.gif)
155535-54-9
9 suppliers
inquiry

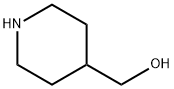
6457-49-4
356 suppliers
$18.00/25g

24424-99-5
871 suppliers
$13.50/25G
![1-[4-(Hydroxymethyl)piperidin-1-yl]-2,2-dimethylpropan-1-one](/CAS/GIF/155535-54-9.gif)
155535-54-9
9 suppliers
inquiry

3282-30-2
380 suppliers
$19.85/100ml
![1-[4-(Hydroxymethyl)piperidin-1-yl]-2,2-dimethylpropan-1-one](/CAS/GIF/155535-54-9.gif)
155535-54-9
9 suppliers
inquiry
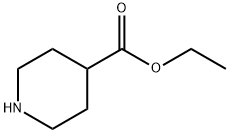
1126-09-6
469 suppliers
$15.40/25g
![1-[4-(Hydroxymethyl)piperidin-1-yl]-2,2-dimethylpropan-1-one](/CAS/GIF/155535-54-9.gif)
155535-54-9
9 suppliers
inquiry