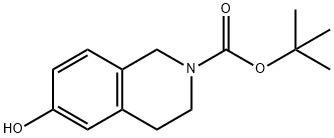
TERT-BUTYL 6-HYDROXY-3,4-DIHYDROISOQUINOLINE-2(1H)-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 6-HYDROXY-3,4-DIHYDROISOQUINOLINE-2(1H)-CARBOXYLATE
- CAS Number:158984-83-9
- Molecular formula:C14H19NO3
- Molecular Weight:249.31

24424-99-5
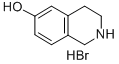
59839-23-5

158984-83-9
The general procedure for the synthesis of N-BOC-6-hydroxy-3,4-dihydroisoquinolines from di-tert-butyl dicarbonate and 1,2,3,4-tetrahydroisoquinolin-6-ol hydrobromide was as follows: 1,2,3,4-tetrahydroisoquinolin-6-ol hydrobromide (1.54 g, 6.4 mmol) was suspended in water, and a solution of tetrahydrofuran was slowly added dropwise with triethylamine (1.42 mg, 14.0 mmol ) and di-tert-butyl dicarbonate (1.67 mg, 7.6 mmol) to a tetrahydrofuran solution. The reaction mixture was stirred at room temperature overnight. Subsequently, the reaction solution was concentrated and the residue was dissolved in ethyl acetate, washed with water and dried over anhydrous sodium sulfate. After removal of the solvent by evaporation under reduced pressure, the residue was purified by fast column chromatography (ethyl acetate: hexane = 1:5) to afford N-BOC-6-hydroxy-3,4-dihydroisoquinoline as a viscous yellow oil (1.58 g, 99%). The product was characterized by 1H NMR (300 MHz, CDCl3) and ESI-MS: 1H NMR (300 MHz, CDCl3) δ 6.92 (s, 1H), 6.71 (s, 1H), 6.70 (s, 1H), 6.31 (s, 1H), 4.48 (s, 2H), 3.60 (t, J=5.7Hz, 2H), 2.75 (t, J=5.7Hz, 2H), 1.49 (s, 9H); ESI-MS m/z 248 (MH-).

24424-99-5
871 suppliers
$13.50/25G

59839-23-5
78 suppliers
$51.00/250mg

158984-83-9
111 suppliers
$57.00/250mg
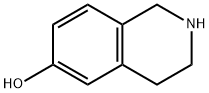
14446-24-3
194 suppliers
$23.00/250mg

24424-99-5
871 suppliers
$13.50/25G

158984-83-9
111 suppliers
$57.00/250mg

24424-99-5
871 suppliers
$13.50/25G
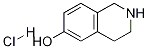
63905-73-7
27 suppliers
inquiry

158984-83-9
111 suppliers
$57.00/250mg

24424-99-5
871 suppliers
$13.50/25G
22246-12-4
117 suppliers
$45.00/50mg

158984-83-9
111 suppliers
$57.00/250mg