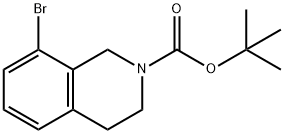
TERT-BUTYL 8-BROMO-3,4-DIHYDROISOQUINOLINE-2(1H)-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 8-BROMO-3,4-DIHYDROISOQUINOLINE-2(1H)-CARBOXYLATE
- CAS Number:893566-75-1
- Molecular formula:C14H18BrNO2
- Molecular Weight:312.2

24424-99-5
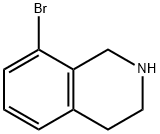
75416-51-2

893566-75-1
Di-tert-butyl dicarbonate (Boc2O, 25.73 g, 117.88 mmol) was added slowly and dropwise at 0 °C to dichloromethane (DCM, containing 8-bromo-1,2,3,4-tetrahydroisoquinoline (Intermediate 21, 25.00 g, 117.88 mmol) and triethylamine (TEA, 32.83 mL, 236.00 mmol) in a 300 mL) solution. The reaction mixture was stirred continuously for 30 minutes at room temperature. Upon completion of the reaction, citric acid solution was added to quench the reaction, followed by a layering operation. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate, 3/1, v/v) to afford N-boc-8-bromo-1,2,3,4-tetrahydroisoquinoline (Intermediate 22, 35 g, 95% yield).

24424-99-5
868 suppliers
$13.50/25G

75416-51-2
95 suppliers
$24.00/100mg

893566-75-1
69 suppliers
$11.00/100mg
Yield: 95%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 0.5 h;
Steps:
A10 Preparation of intermediate 22:
Boc2O (25.73 g, 117.88 mmol) was added dropwise to a solution of intermediate 21 (25.00 g, 117.88 mmol) and TEA (32.83 mL, 236.00 mmol) in DCM (300 mL) at 0 °C. The resulting mixture was stirred at room temperature for 30 minutes. Sat. citric acid was added to quench the reaction and layers were separated. The organic layer was washed with brine, dried over Mg504, filtered and evaporated in vacuo. The crude residue was purified by silica gel column (mobile phase: Petroleum ether/EtOAc, 3/1, v/v) to give 35 g of intermediate 22 (95% yield).
References:
JANSSEN PHARMACEUTICA NV;PILATTE, Isabelle, Noëlle, Constance;QUEROLLE, Olivier, Alexis, Georges;ANGIBAUD, Patrick, René;BERTHELOT, Didier, Jean-Claude;COUPA, Sophie;DEMESTRE, Christophe, Gabriel, Marcel;MEERPOEL, Lieven;MERCEY, Guillaume, Jean, Maurice;MEVELLEC, Laurence, Anne;MEYER, Christophe;PASQUIER, Elisabeth, Thérèse, Jeanne;PONCELET, Virginie, Sophie WO2017/216293, 2017, A1 Location in patent:Page/Page column 74; 75
226942-29-6
152 suppliers
$19.00/250mg

24424-99-5
868 suppliers
$13.50/25G

75416-51-2
95 suppliers
$24.00/100mg

893566-75-1
69 suppliers
$11.00/100mg
893566-74-0
107 suppliers
$28.00/250mg
63927-22-0
264 suppliers
$7.00/250mg

893566-75-1
69 suppliers
$11.00/100mg