
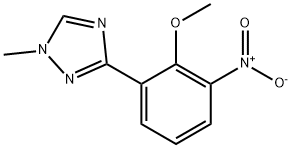
2-methoxy-3-(1-methyl-1H-1,2,4-triazol-3-yl)aniline synthesis
- Product Name:2-methoxy-3-(1-methyl-1H-1,2,4-triazol-3-yl)aniline
- CAS Number:1609394-10-6
- Molecular formula:C10H12N4O
- Molecular Weight:204.23
1609394-08-2
21 suppliers
inquiry

1609394-10-6
165 suppliers
inquiry
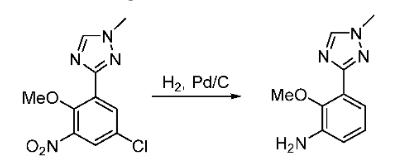
Yield:1609394-10-6 95.57%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol; for 24 h;
Steps:
296 Synthesis of compound 296.3
To a compound of 296.2 (3g, 12.8mmol, 1.Oeq) in MeOH (30mL) 10% Pd/C (1.5g) was added. Hydrogen was purged through the reaction mixture for 24h. After completion of the reaction, the reaction mixture was filtered through celite bed, washed with MeOH and concentrated in vacuo to obtain crude product. This was purified by column chromatography using 20% ethyl acetate in hexane to obtain pure 296.3 (2.5g, 95.57%). MS(ES):m/z 205.84 [M+H]t
References:
WO2018/75937,2018,A1 Location in patent:Paragraph 001348; 001351
![2-Methoxy-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine](/CAS/20180703/GIF/1609393-90-9.gif)
1609393-90-9
45 suppliers
$130.00/50mg
56616-91-2
108 suppliers
$9.00/100mg

1609394-10-6
165 suppliers
inquiry
22621-41-6
196 suppliers
$10.00/1g

1609394-10-6
165 suppliers
inquiry
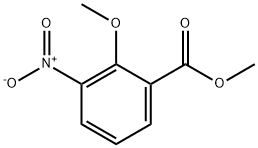
90564-26-4
98 suppliers
$90.00/100mg

1609394-10-6
165 suppliers
inquiry
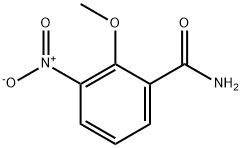
722538-98-9
33 suppliers
inquiry

1609394-10-6
165 suppliers
inquiry