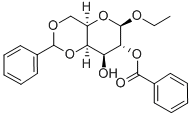
ETHYL 2-O-BENZOYL-4,6-O-BENZYLIDENE-BETA-D-GALACTOPYRANOSIDE synthesis
- Product Name:ETHYL 2-O-BENZOYL-4,6-O-BENZYLIDENE-BETA-D-GALACTOPYRANOSIDE
- CAS Number:161765-88-4
- Molecular formula:C22H24O7
- Molecular Weight:400.42
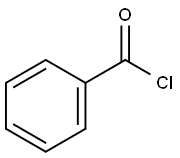
98-88-4
598 suppliers
$10.00/5g
541-88-8
165 suppliers
$10.00/5g

161765-88-4
14 suppliers
$65.00/10g
Yield:161765-88-4 1.7 g (48 %)
Reaction Conditions:
with pyridine;ammonia in methanol;dichloromethane;
Steps:
1 Ethyl 4,6-O-benzylidene-2-O-benzoyl-β-D-galactospyranoside (6) and Ethyl 4,6-O-benzylidene-3-O-(trichloroacetyl-N-carbamoyl)-2-O-benzoyl-β-D-galactospyranoside (Compound 7)
EXAMPLE 1 Ethyl 4,6-O-benzylidene-2-O-benzoyl-β-D-galactospyranoside (6) and Ethyl 4,6-O-benzylidene-3-O-(trichloroacetyl-N-carbamoyl)-2-O-benzoyl-β-D-galactospyranoside (Compound 7) Ethyl 4,6-O-benzylidene-β-D-galactopyranoside Compound 5, [Wallenfels et al., Justis Liebigs Ann. Chem., 635:166 (1960)] (2.9 g, 9.8 mmol) was dissolved in CH2 Cl2 (29 mL) and pyridine (5.4 mL) and cooled to -65° C. under argon. Chloroacetic anhydride (0.82 mL, 10.3 mmol) was then added and the reaction mixture was stirred at -65° C. for two hours. The first stage of the reaction was complete at this time, and benzoyl chloride (1.36 mL, 11.78 mmoles) was then added at -65° C. The reaction mixture was permitted to warm to room temperature, stirred overnight (about 18 hours), diluted with CH2 Cl2 (100 mL), and washed with 1M citric acid (100 mL), water (50 mL), saturated NaHCO3 (50 mL), water (50 mL), and saturated NaCl(50 mL). The organic layer was dried (Na2 SO4), filtered and concentrated to afford a crude 2-benzoyl-3-chloroacetyl product. The crude product was then dissolved in methanol (50 mL) and cooled to -30° C. A solution of 2M NH3 in methanol (6.0 mL) was added and the reaction stirred at -30° C. for four hours to selectively cleave the chloroacetyl group. The resulting mixture was poured into CH2 Cl2 (100 mL) and washed with water (100 mL), saturated NaHCO3 (100 mL), water (100 mL), and saturated NaCl (50 mL). The organic solution was dried (Na2 SO4), filtered, concentrated and chromatographed (silica, 80 percent ethyl acetate/hexane) to afford 1.7 g (48 percent) of Compound 6 as a white solid. Rf =0.3 (silica, ethyl acetate/hexane); 1 H NMR (CDCl3) δ8.07 (d, J=7.5 Hz, 2H, arom.), 7.55 (m, 3H, arom.), 7.46 (d, J=7.5 Hz, 2H, arom.), 7.38 (m, 3H, aromatic), 5.59 (s, 1H, benzylidene), 5.36 (dd, J=10.07, 8.2 Hz, 1H, H-2), 4.64 (d, J=8.2 Hz, 1H, H-1), 4.39 (dd, J=12.5, 1.1 Hz, 1H, H-6), 4.28 (d, J=4.0 Hz, 1H, H-4), 4.12 (dd, J=1.5, 12.5 Hz, 1H, H-6), 3.99-3.86 (multiple peaks, 2H, OCH2 CH3, H-3), 3.61 (m, 1H, OCH2 CH3), 3.56 (bs, 1H, H-5), 2.63 (d, J=9.0 Hz, 1H, OH), 1.21 (t, 3H, CH3).
References:
US5559103,1996,A