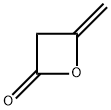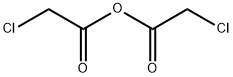
Chloroacetic anhydride synthesis
- Product Name:Chloroacetic anhydride
- CAS Number:541-88-8
- Molecular formula:C4H4Cl2O3
- Molecular Weight:170.97
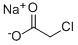
3926-62-3
300 suppliers
$16.69/250g

79-04-9
382 suppliers
$12.00/5g

541-88-8
165 suppliers
$10.00/5g
Yield:541-88-8 93%
Reaction Conditions:
in toluene at 40; for 3 h;Solvent;Temperature;
Steps:
4.1; 4.2 Example 4
Step one, preparation of chloroacetic anhydride solution: adding 1 equivalent of sodium chloroacetate to the reaction flask, and then adding aprotic solvent, the weight of the aprotic solvent is twice that of sodium chloroacetate, heating to 40 °C, stirring, slowly dropping Add 0.9 equivalent of chloroacetyl chloride, complete the dropwise addition for 2 hours, continue to stir at 40 °C for 1 hour, filter, filter cake washed with aprotic solvent, filter, and combine the filtrate to obtain a solution of chloroacetic anhydride with a purity of 95.2%. , the yield is 95.4%, wherein the aprotic solvent is toluene; Step 2: Purification of chloroacetic anhydride: Add the above chloroacetic anhydride solution to the reactor, add petroleum ether, cool to 35 °C, add 0.001 equivalent of chloroacetic anhydride solid as a crystal nucleus and continue to stir until the solid precipitates and cool down to 0. The mixture was stirred for 1 hour at °C, filtered, and the filter cake was washed with petroleum ether. The filter cake was dried at 25 °C to obtain a white crystalline solid product which was obtained as a chloroacetic acid anhydride with a yield of 93 % and a purity of 99.5 %.
References:
Shanghai Renshi Pharmaceutical Technology Co., Ltd.;Li Wenjie;Song Dong;Lin Changxue CN108191643, 2018, A Location in patent:Paragraph 0018-0031
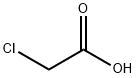
79-11-8
0 suppliers
$24.00/25g

541-88-8
165 suppliers
$10.00/5g
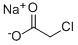
3926-62-3
300 suppliers
$16.69/250g

541-88-8
165 suppliers
$10.00/5g

79-04-9
382 suppliers
$12.00/5g

541-88-8
165 suppliers
$10.00/5g