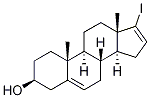
17-Iodoandrosta-5,16-dien-3beta-ol synthesis
- Product Name:17-Iodoandrosta-5,16-dien-3beta-ol
- CAS Number:32138-69-5
- Molecular formula:C19H27IO
- Molecular Weight:398.32
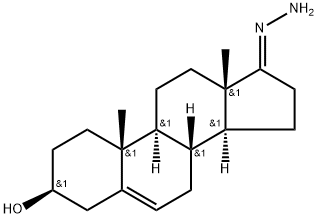
63015-10-1

32138-69-5
As an example, the synthesis of 17-iodo-androsta-5,16-dien-3β-ol (13) was carried out: iodine (12.16 g, 0.0203 mol) was dissolved in anhydrous THF (144 mL) and dry Et2O (72 mL), stirred and cooled to 0 °C in an ice bath. Subsequently, 1,1,3,3-tetramethylguanidine (6.72 mL, 6.24 g, 0.054 mol) was added to this solution. A THF (81 mL) solution of compound 12 (3.0 g, 9.9 mmol) was slowly added dropwise to the above iodine solution over a period of 2 hrs, ensuring that the reaction temperature was maintained at 0°C. Upon completion of the reaction, the mixture was concentrated under vacuum, cooled to ice bath temperature and then dried under vacuum at room temperature to give the yellow solid product 13 (3.65 g, 92.4% yield). The melting point of the product was 169-171 °C (literature value 175-176 °C); IR (CHCl3) showed absorption peaks located at 2935, 1371, 1039, 862, 843, 799, 715, 665, 582 and 566 cm-1 ; 1H NMR (300 MHz, CDCl3) δ value: 0.76 (s, 3H, 18-CH3), and 1.05 (s, 3H, 19-CH3), 3.50 (br s, 1H, 3α-H), 5.35 (s, 1H, 6-H) and 6.14 (s, 1H, 16-H).

63015-10-1
44 suppliers
inquiry

32138-69-5
214 suppliers
$28.60/1MG
Yield:32138-69-5 92.4%
Reaction Conditions:
with iodine;N,N,N',N'-tetramethylguanidine in tetrahydrofuran;diethyl ether at 0; for 2 h;
Steps:
17-Iodoandrosta-5,16-diene-3β-ol (13): A stirred solution of iodine (12.16 g, .0203 mol) in dry of THF (144 mL) and dry OfEt2O (72 mL) was cooled in an ice bath to 0 0C and the solution was treated with 1,1,3,3, tetramethylguanidine (6.72 mL, 6.24g, .054 mole). A solution of compound 12 (3.0 g, 9.9 mmol) in THF (81 mL) was added dropwise to the iodine solution over 2 h maintaining the reaction temperature at 0 0C. The reaction mixture was then concentrated under vacuum, cooled in an ice-bath, and then dried to under vacuum at roomtemperature to afford a yellow solid (13, 3.65 g, 92.4%). mp: 169-171 0C. (lit. 175-176 0C);22 IR (CHCl3) 2935, 1371, 1039, 862, 843, 799, 715, 665, 582, and 566 cm"1; 1HNMR (300MHz, CDCl3): δ 0.76 (s, 3H, 18-CH3), 1.05 (s, 3H, 19-CH3), 3.50 (br s, IH, 3α-H), 5.35 (s,IH, 6-H) and 6.14 (s, IH, 16-H).
References:
WO2006/93993,2006,A1 Location in patent:Page/Page column 31; 45
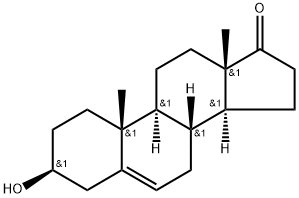
53-43-0
607 suppliers
$27.00/10g

32138-69-5
214 suppliers
$28.60/1MG
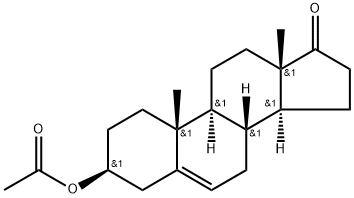
853-23-6
316 suppliers
$28.00/5g

32138-69-5
214 suppliers
$28.60/1MG
122914-94-7
19 suppliers
inquiry

32138-69-5
214 suppliers
$28.60/1MG