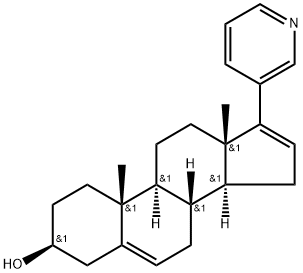
Abiraterone synthesis
- Product Name:Abiraterone
- CAS Number:154229-19-3
- Molecular formula:C24H31NO
- Molecular Weight:349.52
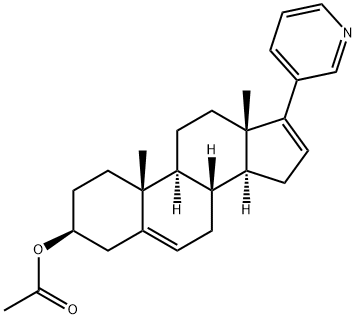
154229-18-2
563 suppliers
$28.00/1unit

154229-19-3
413 suppliers
$28.00/1mg
Yield:154229-19-3 99%
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;water at 0 - 20; for 48 h;Inert atmosphere;
Steps:
Preparation of abiraterone from abiraterone acetate:
To a stirred solution of abiraterone acetate (10 gm, 25.54 mmol) in H2O:THF 1: 1 (102 mL) at 0°C, under nitrogen atmosphere, lithium hydroxide monohydrate was added (3.2 gm, 76.62 mmol). The reaction temperature was gradually raised to RT and stirred for two days. After completion of the reaction the reaction mass was concentrated under vacuum, diluted with water (51 mL), and solids were filtered and dried under high vacuum to obtained the 10,13-dimethyl-17-pyridin-3-yl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol.Yield (8.91 gm, 99 %). 1H - NMR (400 MHz, DMSO-D6): δ 8.43 (d, J = 4.0 Hz, 1H), 7.76 - 7.74 (d, J = 7.6 Hz, 1H), 7.34 - 7.31 (t, J = 4.8 Hz, 1H), 6.11 (s, 1H), 5.30 (s, 1H), 4.60 (s, 1H), 3.31 (bs, 1H), 2.19 - 1.98 (m, 6H), 1.78 - 1.50 (m, 7H), 1.41 - 1.35 (m, 2H), 1.01 - 0.96 (m, 8H); Mass (m/z); 350.1 (M+H)+.
References:
WO2021/100019,2021,A1 Location in patent:Page/Page column 21-22
1421704-60-0
2 suppliers
inquiry

154229-19-3
413 suppliers
$28.00/1mg
32138-69-5
215 suppliers
$28.60/1MG
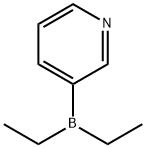
89878-14-8
400 suppliers
$6.00/1g

154229-19-3
413 suppliers
$28.00/1mg

32138-69-5
215 suppliers
$28.60/1MG
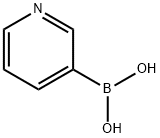
1692-25-7
486 suppliers
$5.00/1g

154229-19-3
413 suppliers
$28.00/1mg
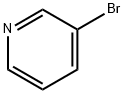
626-55-1
573 suppliers
$5.00/5/ G
![N-[(3-hydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-ylidene)amino]-4-methyl-benzenesulfonamide](/CAS/20180601/GIF/34988-34-6.gif)
34988-34-6
0 suppliers
inquiry

154229-19-3
413 suppliers
$28.00/1mg