
B-[2-[[(3,4-dimethyl-5-isoxazolyl)[(2-methoxyethoxy)methyl]amino]sulfonyl]phenyl] synthesis
- Product Name:B-[2-[[(3,4-dimethyl-5-isoxazolyl)[(2-methoxyethoxy)methyl]amino]sulfonyl]phenyl]
- CAS Number:176961-13-0
- Molecular formula:C15H21BN2O7S
- Molecular Weight:384.21
![2-broMo-N-(3,4-diMethyl-5-isoxazolyl)-N-[(2-Methoxyethoxy)Methyl]benzenesulfonaMide](/CAS/20150408/GIF/153624-30-7.gif)
153624-30-7
2 suppliers
inquiry
![B-[2-[[(3,4-dimethyl-5-isoxazolyl)[(2-methoxyethoxy)methyl]amino]sulfonyl]phenyl]](/CAS/20200611/GIF/176961-13-0.gif)
176961-13-0
7 suppliers
inquiry
Yield:176961-13-0 38.6%
Reaction Conditions:
Stage #1: 2-bromo-N-(3,4-dimethyl-5-isoxazolyl)-N-[(2-methoxyethoxy)methyl]benzenesulfonamidewith n-butyllithium;boric acid tributyl ester in tetrahydrofuran;toluene at -40 - -20;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;water;toluene at -20 - 20;
Steps:
4.1.5. 2-Borono-N-(3,4-dimethyl-5-isoxazolyl)-N-(methoxy-ethoxy-methyl)benzenesulfonamide (11)
At -40 °C and under nitrogen, n-BuLi (4.8 mL, 11.8 mmol) was added dropwise to the mixture of toluene (13 mL), THF (8 mL), tributyl borate (3.2 mL, 11.8 mmol) and compound 10 (3.30 g, 7.9 mmol). The reaction mixture was stirred for 30 min, then its temperature increased slowly to -20 °C. 2 N hydrochloric acid (8 mL) was added and the temperature was rised to room temperature. The water layer was basified to pH 7 with sodium hydroxide solution and extracted with THF (3 × 10 mL). The combined organic extracts were concentrated and purified by flash column chromatography with petroleum ether/EtOAc (1:3, v/v) to afford a red mucoid material in yield of 38.6%. This material was used without further purification.
References:
Bai, Renren;Wei, Zhen;Liu, Jie;Xie, Weijia;Yao, Hequan;Wu, Xiaoming;Jiang, Jieyun;Wang, Qiujuan;Xu, Jinyi [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 15,p. 4661 - 4667] Location in patent:experimental part
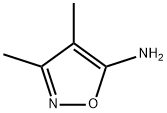
19947-75-2
136 suppliers
$14.14/250mgs:
![B-[2-[[(3,4-dimethyl-5-isoxazolyl)[(2-methoxyethoxy)methyl]amino]sulfonyl]phenyl]](/CAS/20200611/GIF/176961-13-0.gif)
176961-13-0
7 suppliers
inquiry

153623-86-0
0 suppliers
inquiry
![B-[2-[[(3,4-dimethyl-5-isoxazolyl)[(2-methoxyethoxy)methyl]amino]sulfonyl]phenyl]](/CAS/20200611/GIF/176961-13-0.gif)
176961-13-0
7 suppliers
inquiry
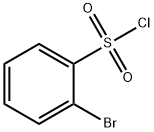
2905-25-1
274 suppliers
$12.00/1g
![B-[2-[[(3,4-dimethyl-5-isoxazolyl)[(2-methoxyethoxy)methyl]amino]sulfonyl]phenyl]](/CAS/20200611/GIF/176961-13-0.gif)
176961-13-0
7 suppliers
inquiry
