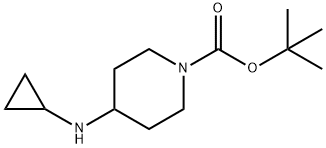
1-TERT-BUTOXYCARBONYL-4-(CYCLOPROPYLAMINO)PIPERIDINE synthesis
- Product Name:1-TERT-BUTOXYCARBONYL-4-(CYCLOPROPYLAMINO)PIPERIDINE
- CAS Number:179557-01-8
- Molecular formula:C13H24N2O2
- Molecular Weight:240.34

79099-07-3
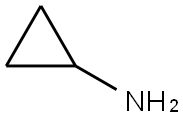
765-30-0

179557-01-8
A methanol (40 mL) solution of tert-butyl 4-oxopiperidine-1-carboxylate (5.0 g, 25.1 mmol) was added to the reaction flask, followed by cyclopropylamine (1.4 g, 25.1 mmol), triethylamine (10.0 mL, 75.3 mmol) and zinc chloride (0.3 g, 2.5 mmol). The reaction mixture was stirred at 60 °C for 7 h. Then sodium cyanoborohydride (4.8 g, 75.3 mmol) was added in batches. The reaction mixture was continued to be stirred at 25 °C for 17 hours. Upon completion of the reaction, the solvent was removed under vacuum and the residue was partitioned between water (250 mL) and ethyl acetate (200 mL). The aqueous layer was further extracted with ethyl acetate (2 x 200 mL) and the organic layers were combined, dried over sodium sulfate and concentrated under vacuum. The residue was purified by column chromatography (basic activated alumina, eluent: 10% to 30% hexane solution of ethyl acetate) to afford the target product tert-butyl 4-(cyclopropylamino)piperidine-1-carboxylate (5.3 g, 88% yield) as a gel. The product data are shown in Table 2.

79099-07-3
0 suppliers
$5.00/5g

765-30-0
507 suppliers
$10.00/25g

179557-01-8
70 suppliers
$45.00/50mg
Yield:179557-01-8 88%
Reaction Conditions:
Stage #1: N-tert-butyloxycarbonylpiperidin-4-one;Cyclopropylaminewith triethylamine;zinc(II) chloride in methanol at 60; for 7 h;
Stage #2: with sodium cyanoborohydride in methanol at 25; for 17 h;
Steps:
To Intermediate 1, tert-butyl 4-oxopiperidine-1-carboxylate (5.0 g, 25.1 mmol) in MeOH (40mL) was added Intermediate 7, cyclopropanamine (1.4 g, 25.1 mmol), Et3N (10.0 mL, 75.3mmol) and ZnCI2 (0.3 g, 2.5 mmol). The reaction mixture was stirred at 60 °C for 7 h, then NaBH3CN (4.8 g, 75.3 mmol) was added portionwise. The resulting reaction mixture was stirred at 25 °C for 17 h. The solvents were removed in vacuo, and the residue was partitioned between H20 (250 mL) and EtOAc (200 mL). The aqueous layer was extracted further withEtOAc (2 x 200 mL), the combined organic layers were dried (Na2SO4) and the solvent was removed in vacuo. The residue was purified by column chromatography (normal basic activated alumina, 10 % to 30 % EtOAc in hexane) to give Intermediate 11, tert-butyl 4- (cyclopropylamino)piperidine-1-carboxylate (5.3 g, 88 %) as a gum.The data for Intermediate 11 are in Table 2.
References:
WO2017/21730,2017,A1 Location in patent:Page/Page column 46
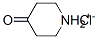
41979-39-9
0 suppliers
$15.00/10mg

179557-01-8
70 suppliers
$45.00/50mg

24424-99-5
871 suppliers
$13.50/25G

179557-01-8
70 suppliers
$45.00/50mg