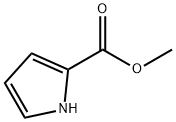
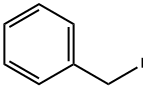
1H-Pyrrole-2-carboxylic acid, 1-(phenylmethyl)-, methyl ester synthesis
- Product Name:1H-Pyrrole-2-carboxylic acid, 1-(phenylmethyl)-, methyl ester
- CAS Number:18159-23-4
- Molecular formula:C13H13NO2
- Molecular Weight:215.25

100-39-0
425 suppliers
$10.00/10g

18159-23-4
6 suppliers
inquiry
Yield:18159-23-4 56%
Reaction Conditions:
Stage #1: methyl Pyrrole-2-carboxylatewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.333333 h;
Stage #2: benzyl bromide in N,N-dimethyl-formamide at 20; for 2 h;
Stage #3: with water;ammonium chloride in N,N-dimethyl-formamide;
Steps:
3.1.a
3.1.a) Synthesis of 1-benzyl-1H-pyrrole-2-carboxaldehyde To a cooled (0° C.) solution of methyl-2-pyrrole carboxylate (8.00 g, 63.9 mmol) in DMF (320 mL) was added NaH (60% by weight 5.10 g, 128 mmol). After 20 min, benzylbromide (11.4 mL, 95.9 mmol) was added and the reaction was warmed to rt. Stirring was continued for 2 h before quenching with saturated aq NH4Cl (0.5 L). The mixture was extracted 3* with EtOAc and the combined organic layers were washed with H2O (3*) and brine, dried over MgSO4, filtered and concentrated in vacuo to give a yellow oil. The crude product was chromatographed over silica gel (0 to 10% EtOAc in heptane over 25 min) to give methyl 1-benzyl-1H-pyrrole-2-carboxylate as a colorless oil (7.75 g, 56%). Rf=0.48 (25:75 heptane/EtOAc); 1H NMR (400 MHz, CDCl3) δ (ppm) 7.28-7.34 (m, 2H) 7.23-7.27 (m, 1H) 7.09-7.13 (m, 2H) 7.01 (dd, J=4.0, 1.8 Hz, 1H) 6.88-6.91 (m, 1H) 6.19 (dd, J=4.0, 2.6 Hz, 1H) 5.57 (s, 2H).
References:
US2008/4327,2008,A1 Location in patent:Page/Page column 73