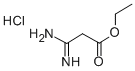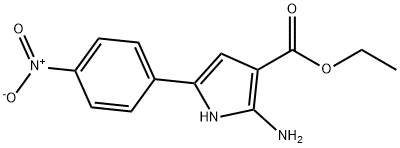
1H-Pyrrole-3-carboxylic acid, 2-aMino-5-(4-nitrophenyl)-, ethyl ester synthesis
- Product Name:1H-Pyrrole-3-carboxylic acid, 2-aMino-5-(4-nitrophenyl)-, ethyl ester
- CAS Number:187724-88-5
- Molecular formula:C13H13N3O4
- Molecular Weight:275.26
99-81-0
222 suppliers
$9.00/1g

187724-88-5
7 suppliers
inquiry
Yield:187724-88-5 79.5%
Reaction Conditions:
Stage #1: carbethoxyacetamidine hydrochloridewith sodium ethanolate in ethanol at 0 - 5; for 0.333333 h;
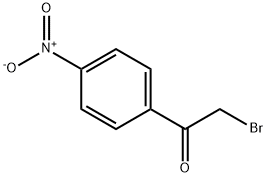
Stage #2: 4-Nitrophenacyl bromide in ethanol at 20; for 24 h;
Steps:
14.14a
Under a nitrogen atmosphere, compound 104(16.7 g, 100 mmol) was introduced into 25 mL of ethanol at 0-5 °C followed by sodium ethanolate (6.8 g, 100 mmol). The yellow suspension was stirred for 20 minutes and compound 501 (12.2 g, 50 mmol) was added. The resulting mixture was stirred for 24 hours at room temperature and
References:
WO2008/33745,2008,A2 Location in patent:Page/Page column 79-80