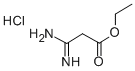
3-Amino-3-iminopropanoic acid ethyl ester hydrochloride synthesis
- Product Name:3-Amino-3-iminopropanoic acid ethyl ester hydrochloride
- CAS Number:57508-48-2
- Molecular formula:C5H11ClN2O2
- Molecular Weight:166.61
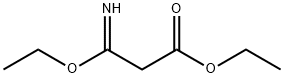
27317-59-5

57508-48-2
The general procedure for the synthesis of ethyl 3-amidinopropionate hydrochloride from ethyl 3-ethoxy-3-iminopropionate was as follows: ethyl 3-amino-3-ethoxyacrylate (760 mg, 4.78 mmol) and ammonium chloride (270 mg, 5.0 mmol) were dissolved in ethanol (4.0 mL) in a glass tube rated for 10 mL pressure. The reaction was carried out using a CEM Discover microwave synthesizer at an initial magnetron power of 200 W for 1 h at 120°C (hold time). Upon completion of the reaction, the reaction mixture was cooled in a stream of compressed air and subsequently filtered. The filtrate was concentrated by vacuum evaporation and the residue was ground with diethyl ether to afford ethyl 3-amidinopropionate hydrochloride (923 mg, 78% yield) as a colorless solid, which was ready for use without further purification. The product characterization data were as follows: νmax (neat)/ cm-1 3300, 3095, 1738, 1687; δH (500 MHz; d6-DMSO)/ ppm 9.16 (2H, broad single peak, NH2), 8.88 (2H, broad single peak, NH2), 4.15 (2H, quartet peak, J = 7.1 Hz, OCH2), 3.63 (2H, single peak, CH2), 1.22 (3H, triple peak, J = 7.1 Hz, CH3); δC (125 MHz, d6-DMSO)/ ppm 166.7 (C=O), 163.6 (C=N), 61.9 (OCH2), 38.2 (CH2), 14.4 (CH3); m/z (EI) 130 (M-+).

27317-59-5
23 suppliers
$250.00/5g

57508-48-2
134 suppliers
$12.00/1g
Yield:57508-48-2 78%
Reaction Conditions:
with ammonium chloride in ethanol at 120; for 1 h;Microwave irradiation;
Steps:
ethyl 3-amino-3-iminopropionate hydrochloride (6*HCl)
A solution of ethyl 3-amino-3-ethoxypropenoate 10 (760 mg, 4.78 mmol) and NH4Cl (270 mg, 5.0 mmol) in EtOH (4.0 mL) was irradiated in a pressure-rated glass tube (10 mL) at 120 °C for 1 h (hold-time) using a CEM Discover microwave synthesizer by moderation of the initial magnetron power (200 W). After cooling in a flow of compressed air, the solution was filtered, evaporated in vacuo and triturated with diethylether to give the title compound1 (923 mg, 78%) as a colourless solid, which was used without further purification; νmax (neat) /cm-1 3300, 3095,1738, 1687; δH (500 MHz; d6-DMSO) /ppm 9.16 (2H, bs, NHH), 8088 (2H, bs, 2H, NHH), 4.15 (2H, q, J = 7.1 Hz, OCH2), 3.63 (2H, s, 2-H),1.22 (3H, t, J = 7.1 Hz, Me); δC (125 MHz, d6-DMSO) /ppm 166.7 (C), 163.6 (C), 61.9 (CH2), 38.2 (CH2), 14.4 (Me); m/z (EI) 130 (M•+).
References:
Bagley, Mark C.;Alnomsy, Ayed;Temple, Scott J. [Synlett,2016,vol. 27,# 11,art. no. ST-2016-D0098-L,p. 1728 - 1732] Location in patent:supporting information
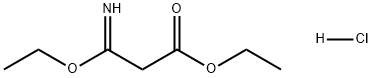
2318-25-4
185 suppliers
$10.00/5g

57508-48-2
134 suppliers
$12.00/1g

105-56-6
460 suppliers
$10.00/5ml

57508-48-2
134 suppliers
$12.00/1g

67-56-1
790 suppliers
$7.29/5ml-f
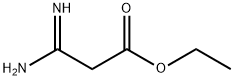
50551-10-5
32 suppliers
$677.00/100g

57508-48-2
134 suppliers
$12.00/1g
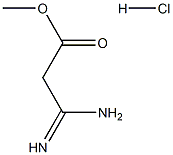
103173-54-2
25 suppliers
$65.00/50mg