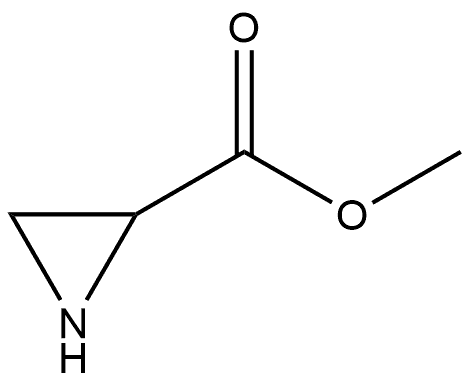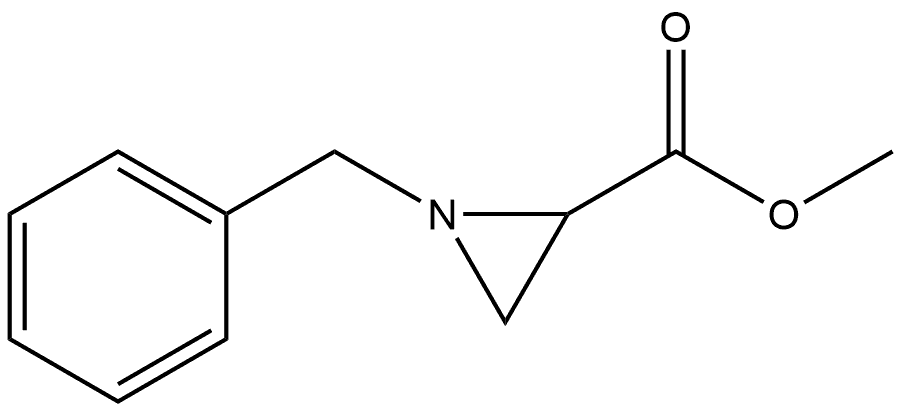
2-Aziridinecarboxylic acid, 1-(phenylmethyl)-, methyl ester, (1R-trans)- (9CI) synthesis
- Product Name:2-Aziridinecarboxylic acid, 1-(phenylmethyl)-, methyl ester, (1R-trans)- (9CI)
- CAS Number:196965-62-5
- Molecular formula:C11H13NO2
- Molecular Weight:191.23
Yield:196965-62-5 97%
Reaction Conditions:
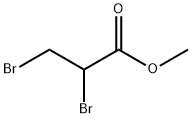
Stage #1: Methyl 2,3-dibromopropionatewith triethylamine in methanol at 0; for 0.25 h;
Stage #2: benzylamine in methanol at 20;
Steps:
6.1.3. Methyl 1-benzylaziridine-2-carboxylate 17
A solution of methyl 2,3-dibromopropanoate 15 at 0 °C (5.01 g, 20.4 mmol, 1.0 eq.) in methanol (10 ml) was added drop-wise to astirred solution of triethylamine (6.18 g, 8.47 ml, 61.1 mmol, 3.0 eq.)in methanol (40 ml) and stirred for 15 min. Benzylamine (2.18 g,2.22 ml, 20.4 mmol, 1.0 eq.) in methanol (40 ml) was then added drop-wise and the reaction mixturewas stirred for a further 30 min after which time the solutionwas left towarm to room temperatureand stirred overnight. The reaction mixture was evaporated invacuo, diluted with diethyl ether (100 ml) and washed with water(200 ml). The aqueous phase was extracted with diethyl ether(3 50 ml). The combined organic phases were dried (Na2SO4),filtered, and evaporated in vacuo. The resulting material was purifiedby column chromatography (25% ethyl acetate: hexane) toafford methyl 1-benzylaziridine-2-carboxylate 17 as a yellow oil(3.79 g, 97%). Rf 0.27 (25% ethyl acetate: hexane); νmax (neat)/cm-11737, 1442, 1180, 1028; 1H NMR (300 MHz, CDCl3); δ 7.39e7.13 (m,5H, ArH's), 3.70 (s, 3H, OCH3), 3.53 (s, 2H, CH2Ph), 2.25 (dd, 1H,J 1.1 &3.1 Hz, CHN), 2.20 (dd, 1H, J 3.1 &6.4 Hz, CHCH2aN), 1.74(dd, 1H, J 1.0 & 6.4 Hz, CHCH2bN); 13C NMR (75 MHz, CDCl3);δ 174.40, 138.04, 128.49, 128.12, 127.42, 63.98, 52.22, 37.40, 34.57;HRMS m/z (ESI) 192.1058 ([M + H]+ requires 192.1026).
References:
van Greunen, Divan G.;Cordier, Werner;Nell, Margo;van der Westhuyzen, Chris;Steenkamp, Vanessa;Panayides, Jenny-Lee;Riley, Darren L. [European Journal of Medicinal Chemistry,2017,vol. 127,p. 671 - 690]
53386-64-4
43 suppliers
$10.00/250mg

196965-62-5
0 suppliers
inquiry