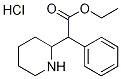
2-Piperidineacetic acid, α-phenyl-, ethyl ester, hydrochloride (1:1) synthesis
- Product Name:2-Piperidineacetic acid, α-phenyl-, ethyl ester, hydrochloride (1:1)
- CAS Number:19716-79-1
- Molecular formula:C15H22ClNO2
- Molecular Weight:283.7937

64-17-5
758 suppliers
$10.00/50g
19395-39-2
77 suppliers
$24.73/1gm:

19716-79-1
0 suppliers
$215.61/1gm:
Yield:-
Reaction Conditions:
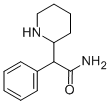
Stage #1:ethanol;(±)-erythro/threo-2-phenyl-2-(piperidin-2-yl)acetamide with sulfuric acid at 2 - 95;
Stage #2: with hydrogenchloride in isopropyl alcohol at 5 - 65;
Steps:
Preparation of ethylphenidate hydrochloride from threo-2-phenyl-2-piperidyl acetamide:
In ethanol (500 ml), threo-2-phenyl-2-(piperidine-2-yl)acetamide (100 g, 1.0 mole) was added at ambient temperature.The reaction mixture was cooled to 2-8°C followed by drop wise addition of sulfuric acid (224.4 g, 5.0 mole) over 30-40 min. The solid mass obtained was stirred for 15-20min at 5-10°C and temperature was then raised up to room temperature. The reaction mass was further heated at temperature 80 to 95°C for 15-30 min and maintained at reflux for 30 h to distil some volume of ethanol followed by adding fresh ethanol into the reaction mass. Further it is maintained at reflux for 5 h. After completion of reaction, ethanol was distilled off and degassed under vacuum. The thick residue was cooled to 20-25°C and water (1000 ml) was added.Cooled the mass up at 10-15°C and stirred for 10 min followed by adjusting its pH at 6-8 by adding caustic soda. Into a reaction mixture, dichloromethane (200 ml) was added at 25-30°C and pH was adjusted to 11.0-12.5. The mixture was then stirred for 30 min at 25-30°C. The organic layer was separated. Again dichloromethane (75 ml x2) was added to aqueous layer and separated. Dichloromethane was distilled out at temperature 45-50°C and degassed under vacuum. After completion of distillation, isopropanol (800 ml)added into the oily mass followed by charcoal treatment. The reaction mixture was filtered and washed with isopropanol(50 ml). The filtrate was then cooled up to 5-10°C.lsopropanolic hydrochloric acid (80 g, 1.1 mole) was added to the reaction mass followed by stirring for 30 min at 5-10°C. The reaction mixture was heated at 55-65°C for 5-10min followed by cooling at 0-5°C. The reaction mass was maintained at 0-8°C for 30 min. Filtered the mass at 0-8°C and washed with isopropanol (50 ml). The wet cake was dried at 40-45°C under vacuum to get ethylphenidate hydrochloride(92.0 g, 81.5%) having HPLC purity: Threo content:99.9%, Erythro content: 0.10%.
References:
Aswar, Anand S.;Tazeenuddin, Mubashshir A. [Journal of the Indian Chemical Society,2018,vol. 95,# 6,p. 635 - 640]