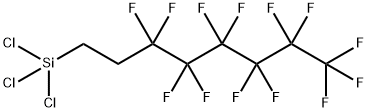
1H,1H,2H,2H-Perfluorooctyltrichlorosilane synthesis
- Product Name:1H,1H,2H,2H-Perfluorooctyltrichlorosilane
- CAS Number:78560-45-9
- Molecular formula:C8H4Cl3F13Si
- Molecular Weight:481.54
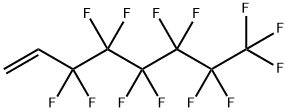
25291-17-2
212 suppliers
$15.00/5G

78560-45-9
215 suppliers
$12.00/1g
Yield:78560-45-9 2.6%
Reaction Conditions:
with trichlorosilane;platinum 1,3-divinyl-1,1,3,3-tetramethyldisiloxane in xylene at 86 - 126; under 760.051 - 5625.56 Torr; for 4.5 - 10.5 h;Product distribution / selectivity;
Steps:
1; 2; 1; 2; 3
Comparative example 1 120 g of 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctene (iodine content = 170 ppm by weight) and 0. 1733 g of CPC072 (Pt (0)-divinyltetramethyldisiloxane in xylene, containing 2% by weight of Pt), corresponding to a molar ratio of Pt to fluoroolefin of 1: 20 000, are placed in a 250 ml stirred apparatus provided with a dropping funnel and, at ambient pressure, 52 g of trichlorosilane are metered in under nitrogen over the reaction time of 6 hours. The reaction temperature is from 86 to 93°C. The yield of trichlor (3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctyl) silane after the end of the reaction, based on the 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctene used, is 2. 60/, >.; Comparative example 2 120 g of 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctene (iodine content = 6. 5 ppm by weight) and 0. 1733 g of CPC072 (Pt (0)-divinyltetramethyidisiloxane in xylene, containing 2% by weight of Pt), corresponding to a molar ratio of Pt to fluoroolefin of 1: 20 000, are placed in a 250 ml stirred apparatus provided with a dropping funnel and, at ambient pressure, 52 g of trichlorosilane are metered in under nitrogen over the reaction time of 4.7 hours. The reaction temperature is from 86 to 93°C. The yield of trichlor (3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctyl) silane after the end of the reaction, based on the 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctene used, is 87%.; Example 1 100 g of trichlorosilane are placed in a 350 ml steel autoclave at room temperature, 0.35 g of CPC072 (Pt (0)-divinyltetramethyldisiloxane in xylene, containing 2% by weight of Pt) is added under a blanket of nitrogen and 221.4 g of 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8- tridecafluorooctene (iodine content = 170 ppm by weight), corresponding to a molar ratio of Pt to fluoroolefin of 1: 20 000, are subsequently metered in at an internal reactor pressure of from 5.8 to 6 bar abs. over a period of 4.5 hours by means of a pump. The reaction temperature is set to from 116 to 126°C at about 3 bar abs. The yield of trichloro- (3, 3,4, 4,5, 5,6, 6,7, 7,8, 8, 8-tridecafluorooctyl) silane after the end of the reaction, based on the 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctene used, is 82%.; Example 2 100 g of trichlorosilane are placed in a 350 ml steel autoclave at room temperature, heated and 0.35 g of CPC072 (Pt (0)-divinyltetramethyldisiloxane in xylene, containing 2% by weight of Pt) are added under a blanket of nitrogen and 242.2 g of 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctene (iodine content = 8 ppm by weight), corresponding to a molar ratio of Pt to fluoroolefin of 1: 20 000, are subsequently metered in at a reactor pressure of from 2 bar to 6 bar abs. over a period of 4 hours by means of a pump. The reaction temperature is from 102 to 116°C. After a time of 6.5 hours, a yield of trichloro (3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctyl) silane, based on the 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctene used, of 94% is achieved. 320 g of oro- (3, 3,4, 4,5, 5,6, 6,7, 7,8, 8, 8-tridecafluorooctyl) silane are firstly placed in a 1 I stirred apparatus provided with a dropping funnel and nitrogen blanketing. This initial charge is heated until the liquid in the apparatus has reached a temperature of about 1 OOqC. 91.8 g of ethanol (denatured with 1% of methyl ethyl ketone) are then metered in over a period of about 4 hours. After the end of the metered addition, the mixture is stirred for about 2 hours more, then cooled before 55. 0 g of (20% strength) sodium ethoxide are added. The yield, based on trichlor (3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8- tridecafluorooctyl) silane, is 91.6%.; Example 3 95 g of trichlorosilane are placed in a 350 mi steel autoclave at room temperature, heated and 0.21 g of CPC072 (Pt (0)-divinyltetramethyldisiloxane in xylene, containing 2% by weight of Pt) are added under a blanket of nitrogen. This corresponds to a molar ratio of Pt to fluoroolefin of 1: 33 000. 242 g of 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8- tridecafluorooctene (iodine content = 6.5 ppm by weight) together with an additional 0.21 g of CPC072 are subsequently metered in at a reactor pressure of from 5.7 bar to 7.5 bar abs. over a period of 3 hours by means of a pump (final ratio of Pt to fluoroolefin = 1: 17 000). The reaction temperature is from 106 to 124°C. After a time of 4.8 hours, a yield of trichlor (3,3, 4, 4, 5, 5,6, 6,7, 7,8, 8, 8-tridecafluorooctyl) silane, based on the 3,3, 4,4, 5,5, 6,6, 7,7, 8,8, 8-tridecafluorooctene used, of 93% is achieved.
References:
WO2005/58919,2005,A1 Location in patent:Page/Page column 8-10

25291-17-2
212 suppliers
$15.00/5G
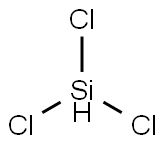
10025-78-2
186 suppliers
$22.50/25g

78560-45-9
215 suppliers
$12.00/1g