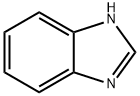
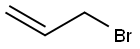
1H-Benzimidazole,1-(2-propenyl)-(9CI) synthesis
- Product Name:1H-Benzimidazole,1-(2-propenyl)-(9CI)
- CAS Number:19018-22-5
- Molecular formula:C10H10N2
- Molecular Weight:158.2
Yield:19018-22-5 97%
Reaction Conditions:
with sodium hydroxide in water at 20; for 0.166667 h;Micellar solution;Reagent/catalyst;
Steps:
2.1 4.1 General procedure for N-alkylation of benzimidazoles and imidazoles
General procedure: To the reaction vessel containing benzimidazole or imidazole (1mmol), alkyl halide (1mmol), 50% NaOH (0.5mL) and anionic surfactant sodium dodecyl sulfate (5mol%) was added and the reaction mixture was vigorously stirred at room temperature or 60°C. After the completion of reaction (TLC) followed by standard workup using ethyl acetate as extracting solvent the crude product was purified over silica-gel (60-120 mesh) using ethyl acetate-hexane (3:7) as eluent to afford pure products. Identities of the products were judged by the comparison of melting point, IR data, 1H NMR, 13C NMR and HRMS analyses. 4.2.1 1-Allyl benzimidazole(3a) [ 51 ] Yield 97%, colorless syrup; IR (KBr) νmax 3392, 2923, 1645, 1615, 1495, 1459?cm-1; 1H NMR (300?MHz, CDCl3) δ 7.82 (s, 1H, -CH=N), 7.75-7.72 (m, 1H, aromatic H), 7.31-7.28 (m, 1H, aromatic H), 7.23-7.20 (m, 1H, aromatic H), 5.99-5.86 (m, 1H, HC=C), 5.21 (d, J?=?10.2?Hz, 1H), 5.10 (d, J?=?17.1?Hz, 1H), 5.14-5.08 (m, 1H), 4.69 (d, J?=?5.4?Hz, 2H); 13C NMR (75?MHz, CDCl3) δ 143.8 (-C=N), 142.9, 133.8, 131.9, 122.9, 122.1, 120.3, 118.6, 109.9, 47.4 (-NCH2).
References:
Chakraborty, Ankita;Debnath, Sudipto;Ghosh, Tanmoy;Maiti, Dilip K.;Majumdar, Swapan [Tetrahedron,2018,vol. 74,# 40,p. 5932 - 5941]
87216-53-3
23 suppliers
$28.60/10MG

19018-22-5
4 suppliers
inquiry