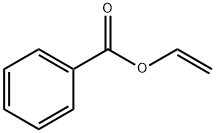
(1H-indol-1-yl)(phenyl)methanone synthesis
- Product Name:(1H-indol-1-yl)(phenyl)methanone
- CAS Number:1496-76-0
- Molecular formula:C15H11NO
- Molecular Weight:221.25
Yield:1496-76-0 99%
Reaction Conditions:
with sodium carbonate in acetonitrile at 120; for 24 h;Schlenk technique;regioselective reaction;
Steps:
Na2CO3-Catalyzed N-Acylation of Indoles; General Procedure
General procedure: A mixture of indole 1 (0.50 mmol), Na2CO3 (10.6 mg, 0.10 mmol, 20 mol%), and alkenyl carboxylate 2 (2.0 mmol, 4.0 equiv) in MeCN (3 mL) was added into a Schlenk flask (25 mL) and stirred at 120 °C until completion of the reaction. Then the solvent was evaporated under reduced pressure and the residue was purified by column chromatography (petroleum ether/EtOAc 20:1 to 5:1) to afford the desired acylindoles 3 (Tables 2 and 3).
References:
Zhou, Xiao-Yu;Chen, Xia [Synthesis,2019,vol. 51,# 2,p. 516 - 521] Location in patent:supporting information
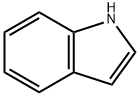
120-72-9
658 suppliers
$6.00/25g
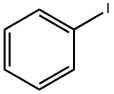
591-50-4
500 suppliers
$10.00/1g
201230-82-2
1 suppliers
inquiry

1496-76-0
6 suppliers
inquiry

120-72-9
658 suppliers
$6.00/25g

108-86-1
523 suppliers
$10.00/5g
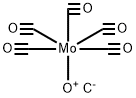
199620-15-0
0 suppliers
inquiry

1496-76-0
6 suppliers
inquiry

120-72-9
658 suppliers
$6.00/25g

108-86-1
523 suppliers
$10.00/5g
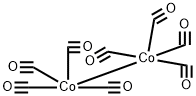
61117-58-6
0 suppliers
inquiry

1496-76-0
6 suppliers
inquiry