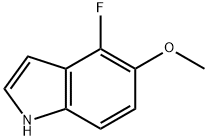
1H-Indole,4-fluoro-5-methoxy-(9CI) synthesis
- Product Name:1H-Indole,4-fluoro-5-methoxy-(9CI)
- CAS Number:288385-89-7
- Molecular formula:C9H8FNO
- Molecular Weight:165.16

67-56-1
781 suppliers
$7.29/5ml-f
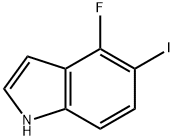
1240113-40-9
38 suppliers
inquiry

288385-89-7
37 suppliers
inquiry
Yield: 23%
Reaction Conditions:
Stage #1:methanol with sodium
Stage #2:4-fluoro-5-iodo-1H-indole with copper(l) iodide in N,N-dimethyl-formamide at 150;Inert atmosphere;
Steps:
4-Fluoro-5-methoxy-1H-indole (6).
The 4-Fluoro-5-iodo-1H-indole (4, 10 mmol, 2.61 g) was dissolved in 20 mL DMF and the solution was added to a sodium methoxide solution prepared from MeOH (45 mL) and sodium metal (200 mmol, 4.6 g). CuI (20 mmol, 3.79 g) was quickly added and the air from the reaction vessel was displaced with argon.
The mixture was held at 150 °C under an argon atmosphere, and the reaction progress was monitored by TLC.
After complete conversion, the mixture was poured into 50 mL of H2O and extracted with CHCl3 (3 * 20 mL) and the combined organic extract was filtered through a 1-cm bed of silicagel, which was further eluted with CHCl3.
The solvent was stripped off, and the crude product was subjected to flash chromatography (SiO2).
Evaporation of the solvent provided a white solid (381 mg, 23% yield). LCMS: MS [M+H+]: 165.83; tR = 2.53 min. 1H NMR (400 MHz, DMSO-d6) δ 11.21 (s, 1H), 7.36 (t, J = 2.8 Hz, 1H), 7.15 (dt, J = 8.7, 0.8 Hz, 1H), 6.98 (t, J = 8.4 Hz, 1H), 6.43 (ddd, J = 3.0, 2.0, 0.9 Hz, 1H), 3.83 (s, 3H).
13C NMR (101 MHz, DMSO-d6) δ 145.1 (d, 1JCF = 243 Hz), 139.34 (d, 3JCF = 9 Hz), 133.96 (d, 3JCF = 11 Hz), 127.20, 118.07 (d, 4JCF = 4 Hz), 111.55, 107.5 (d, 4JCF = 2 Hz), 96.98 (d, 2JCF = 27.5 Hz), 58.55.
References:
Hogendorf, Adam S.;Hogendorf, Agata;Popiołek-Barczyk, Katarzyna;Ciechanowska, Agata;Mika, Joanna;Satała, Grzegorz;Walczak, Maria;Latacz, Gniewomir;Handzlik, Jadwiga;Kieć-Kononowicz, Katarzyna;Ponimaskin, Evgeni;Schade, Sophie;Zeug, Andre;Bijata, Monika;Kubicki, Maciej;Kurczab, Rafał;Lenda, Tomasz;Staroń, Jakub;Bugno, Ryszard;Duszyńska, Beata;Pilarski, Bogusław;Bojarski, Andrzej J. [European Journal of Medicinal Chemistry,2019,vol. 170,p. 261 - 275]

124-41-4
725 suppliers
$12.00/25g
344790-96-1
68 suppliers
$54.00/100mg

288385-89-7
37 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

288385-89-7
37 suppliers
inquiry
387-43-9
321 suppliers
$10.00/1g

288385-89-7
37 suppliers
inquiry