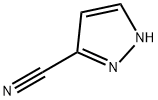
1H-PYRAZOLE-3-CARBONITRILE synthesis
- Product Name:1H-PYRAZOLE-3-CARBONITRILE
- CAS Number:36650-74-5
- Molecular formula:C4H3N3
- Molecular Weight:93.09
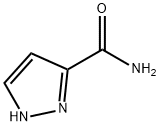
33064-36-7

36650-74-5
The general method for synthesizing 3-carbonitrile pyrazoles from pyrazole-3-carboxamide is as follows: after completion of the preparation of the amide intermediate (Example A), the state of the reaction vessel is determined according to the boiling point of the amide intermediate in relation to the reaction temperature TB: if the boiling point of the amide intermediate at atmospheric pressure is equal to or lower than TB, the reaction vessel is closed; if the boiling point is higher than TB, the reaction vessel is kept open. Subsequently, stirring is continued at a rate of 600 rpm and the reaction temperature is adjusted to TB and maintained at this temperature for TD hours until the reaction is nearly complete. Upon completion of the reaction, the reaction vessel was sealed and connected to a vacuum pump, the vacuum in the reaction vessel was adjusted to 20-50 mbar (depending on the nature of the nitrile product) and the distillate was collected as the nitrile product. The yield of the product was calculated and samples were taken for NMR spectroscopy and elemental analysis to characterize the resulting nitrile product. The specific reaction conditions and characterization results are detailed in the following Tables A-7, A-8, A-9, A-10 and A-11. The characterization results showed that the resulting nitrile product was of very high purity (>99%). In some embodiments of the preparation of the nitrile product, 10 g of phosphorus pentoxide was optionally added as a catalyst at the beginning of the reaction.

33064-36-7
97 suppliers
$69.00/5g

36650-74-5
122 suppliers
inquiry
Yield:36650-74-5 85%
Reaction Conditions:
at 335; for 1 h;Temperature;
Steps:
A Nitrile Product Preparation Example A
General procedure: Following the amide intermediate Preparation Example A. The reaction vessel is closed (when the amide intermediate has a boiling point at normal pressure equal to or lower than the reaction temperature TB described below) or the reaction vessel is kept open (when the amide intermediate has a boiling point higher than the normal pressure When the reaction temperature is TB), the stirring is continued (600 r/min), the reaction temperature is changed to TB, and after the reaction temperature TB is maintained for TD hours, the reaction is almost complete. Then, the reaction vessel was sealed and connected to a vacuum pump so that the degree of vacuum in the reaction vessel reached 20-50 mbar (according to the type of nitrile product) and the distillate was used as the nitrile product. The yield of the nitrile product was calculated and sampled for nuclear magnetic proteomics and elemental analysis to characterize the nitrile product obtained. Specific reaction conditions and characterization results are shown in Tables A-7, A-8, A-9, A-10 and A-11 below. These characterization results show that the nitrile product obtained has an extremely high purity (above 99%).In these nitrile product preparation examples, 10 g of diphosphorus pentoxide was optionally added to the reaction vessel as a catalyst at the start of the reaction.
References:
Sinopec Yangzi Petrochemical Co., Ltd.;Sinopec Corporation;Sun Hailong;Wei Yanyu;Gao Yilong;Chen Xinhua;Miao Jun;Li Na;Kan Lin;Bai Jiye;Chen Shaohui;Yang Aiwu;Xu Yuexing CN104557357, 2018, B Location in patent:Paragraph 0150; 0151; 0152; 0160
30192-58-6
3 suppliers
inquiry

36650-74-5
122 suppliers
inquiry