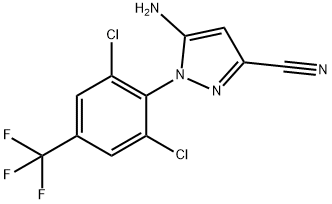
5-Amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole synthesis
- Product Name:5-Amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole
- CAS Number:120068-79-3
- Molecular formula:C11H5Cl2F3N4
- Molecular Weight:321.09
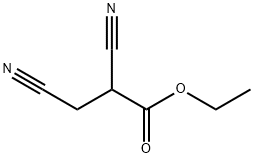
40497-11-8
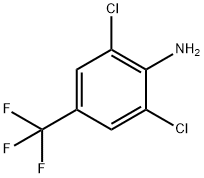
24279-39-8

120068-79-3
Experiment 1: 1. 150 ml of toluene was added to a 2.0 liter four neck glass reactor fitted with an overhead stirrer system. 2. 0.5 g of sucrose polystearate (SP-20) and 230 g of 2,6-dichloro-4-trifluoromethylaniline were added to the reactor under stirring. 3. 600 ml of a 13.9N spent acid solution containing 3.9N sulfuric acid and 10N HCl was added gradually over a period of 30 minutes at 30°C to 40°C. 4. the reaction mixture was stirred at 30 °C to 40 °C for 1 h to form a mixture of 2,6-dichloro-4-trifluoromethylaniline salts and then cooled to 15 °C to 20 °C. the reaction mixture was then stirred at 30 °C to 40 °C for 1 h to form a mixture of 2,6-dichloro-4-trifluoromethylaniline salts. 5. a sodium nitrite solution was prepared by dissolving 75.9 g of sodium nitrite in 100 mL of water. 6. The sodium nitrite solution was added to the reaction mixture over a period of 3 hours at 15°C to 20°C and the diazotization reaction continued with stirring at the same temperature for 1 hour. 7. the reaction mixture was poured into 1200 grams of cold ice water at a controlled temperature below 20°C. 8. 152 g of ethyl 2,3-dicyanopropionate was added to the diluted diazotized substance at 10 °C to 15 °C and stirred for 30 minutes. 9. Stirring was continued for 12 hours at 15°C to 25°C to form a biphasic system containing the coupling product. 10. Stirring was stopped, the organic layer was separated as the coupling product and the aqueous layer was extracted with 100 ml of toluene. 11. The extracted toluene layer was combined with the organic layer and added to 1000 ml of 2N sodium hydroxide solution at 5°C to 25°C and stirred for 4 hours at 20°C to 25°C. 12. The organic phase was heated to 40°C to 42°C and equilibrated for 1 hour. 13. The precipitated product slurry was cooled to 10°C to 15°C, filtered, washed with water until neutral, and then washed with 50 ml of cooled toluene. 14. 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole was isolated in 80% yield and 99.0% HPLC purity. Experiments 2-13: Similar experiments were performed by varying the concentrations of spent HCl and spent H2SO4 and their mixtures, as well as the reaction conditions (Examples 2-13). The results of the experiments are summarized in Table 1.
![Butanedinitrile, 2-[2-[2,6-dichloro-4-(trifluoromethyl)phenyl]hydrazinylidene]-](/CAS/20210305/GIF/213132-03-7.gif)
213132-03-7
0 suppliers
inquiry

120068-79-3
243 suppliers
$10.00/1g
Yield:120068-79-3 97%
Reaction Conditions:
with ammonia in ethanol;water at 0; for 0.166667 h;
Steps:
ii REFERENCE EXAMPLE ii)
Preparation of 5-Amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole
REFERENCE EXAMPLE ii) Preparation of 5-Amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole ammonia (20 microlitres of an 8% ammonia solution in water) was added to a mixture of the above 2-(2,6-dichloro-4-trifluoromethylphenylhydrazono)-succinonitrile (0.077 g) in ethanol (1 ml) and water (0.2 ml) at 0° C. After 10 minutes the mixture was extracted (dichloromethane) and evaporated to give 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole (0.076 g, 97% yield) Purity 98% (by hplc).
References:
US6392081,2002,B1 Location in patent:Page column 9

40497-11-8
222 suppliers
$39.00/5G

24279-39-8
405 suppliers
$6.00/5g

120068-79-3
243 suppliers
$10.00/1g
61760-68-7
1 suppliers
inquiry

24279-39-8
405 suppliers
$6.00/5g

120068-79-3
243 suppliers
$10.00/1g
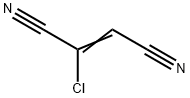
26459-70-1
0 suppliers
inquiry
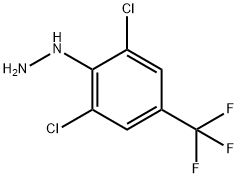
86398-94-9
123 suppliers
$37.00/1g

120068-79-3
243 suppliers
$10.00/1g
![1H-Pyrazole-4-carboxylic acid, 5-amino-3-cyano-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-, ethyl ester](/CAS/20210305/GIF/213457-39-7.gif)
213457-39-7
0 suppliers
inquiry

120068-79-3
243 suppliers
$10.00/1g