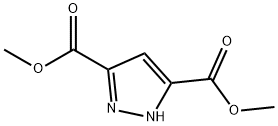
Dimethyl 1H-pyrazole-3,5-dicarboxylate synthesis
- Product Name:Dimethyl 1H-pyrazole-3,5-dicarboxylate
- CAS Number:4077-76-3
- Molecular formula:C7H8N2O4
- Molecular Weight:184.15

67-56-1
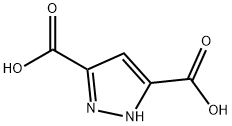
3112-31-0

4077-76-3
The general procedure for the synthesis of dimethyl 1H-pyrazole-3,5-dicarboxylate from methanol and 3,5-pyrazoledicarboxylic acid was as follows: 31.7 g (0.203 mol) of 3,5-pyrazoledicarboxylic acid was mixed with 125 mL of methanol and the mixture was saturated with gaseous HCl. The reaction mixture was heated to reflux for 3 hours and subsequently left to stand overnight at room temperature. After completion of the reaction, the precipitate was collected by filtration and washed with methanol. A final 23.5 g (63% yield) of white crystalline product with a melting point of 142-143 °C was obtained. The structure of the product was confirmed by IR spectroscopy, and the main absorption peaks were 3105 cm-1 (broad peak, NH stretching vibration), 1710 cm-1 (strong peak, C=O stretching vibration), and 1240 cm-1 (strong peak, C-O-C stretching vibration).1H NMR spectroscopic data were as follows: δ 3.82 (single peak, 6H, CH3), δ 6.36 (single peak, 1H, pyrazolyl ring 4-H), δ 14.5 (single peak, 1H, pyrazolyl ring 4-H), and δ 14.5 (single peak, 1H). 4-H), δ 14.43 (single peak, 1H, NH).

67-56-1
790 suppliers
$9.00/25ml

3112-31-0
220 suppliers
$18.00/1g

4077-76-3
124 suppliers
$6.00/100mg
Yield:4077-76-3 99%
Reaction Conditions:
with thionyl chloride at 0 - 80; for 4 h;Inert atmosphere;
Steps:
44 Preparation of Compound 119
In methanol (50 mL), 3,5-pyrazoldicarboxylic acid hydrate (5 g, 28.71 mmol) was dissolved, then thionyl chloride (6.28 mL, 86.15 mmol) was added thereto at 0°C under a nitrogen atmosphere, and then the mixture was heated to 80°C. The reaction solution was stirred for 4 hours and then concentrated to obtain Compound 119 (7.1 g, 99%). 1H-NMR (400 MHz, CDCl3) δ 7.34 (s, 1H), 3.96 (s, 6H) .
References:
EP3604311,2020,A1 Location in patent:Paragraph 0267; 0268

3112-31-0
220 suppliers
$18.00/1g

4077-76-3
124 suppliers
$6.00/100mg
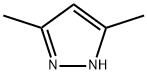
67-51-6
579 suppliers
$6.00/25g

4077-76-3
124 suppliers
$6.00/100mg
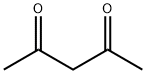
123-54-6
588 suppliers
$10.00/25ml

4077-76-3
124 suppliers
$6.00/100mg
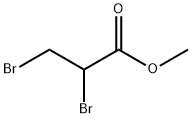
1729-67-5
140 suppliers
$15.00/1g
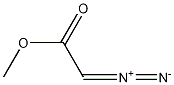
6832-16-2
15 suppliers
inquiry

4077-76-3
124 suppliers
$6.00/100mg