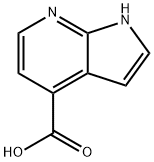
7-Azaindole-4-carboxylic acid synthesis
- Product Name:7-Azaindole-4-carboxylic acid
- CAS Number:479553-01-0
- Molecular formula:C8H6N2O2
- Molecular Weight:162.15
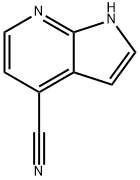
344327-11-3

479553-01-0
General procedure for the synthesis of 7-azaindole-4-carboxylic acid from 4-cyano-7-azaindole: A mixture of 4-cyano-7-azaindole (11.5 g, 80 mmol), sodium hydroxide (32 g, 800 mmol), water (100 mL) and ethanol (100 mL) was heated to reflux. After 6 hours of reaction, the reaction mixture was cooled to room temperature, neutralized and acidified with concentrated hydrochloric acid. The precipitated solid was collected by filtration to afford 7-azaindole-4-carboxylic acid (10.4 g, 80% yield), which was used in subsequent reactions without further purification.

344327-11-3
190 suppliers
$12.00/250mg

479553-01-0
150 suppliers
$24.00/100mg
Yield: 100%
Reaction Conditions:
with ethanol;sodium hydroxide for 20 h;Reflux;
Steps:
2 Preparation Example 2: Preparation of 1H-pyrrole[2,3-b]pyridine-4-carboxylic acid
1H-pyrrole [2,3-b] pyridine-4-carbonitrile obtained in Preparation Example 1 above(100 mg, 0.70 mmol) was dissolved in ethanol (5 ml),Sodium hydroxide (10N, 350 μl, 3.5 mmol) was added and heated to reflux for 20 hours.After the reaction was terminated by adding water (30 ml), Sulfuric acid (93 ul, 1.75 mmol) was added for neutralization, and the resulting sodium sulfate was filtered.Next, the filtrate was concentrated under reduced pressure to obtain the title compound.(114 mg, 0.7 mmol, 100%, colorless oil) was obtained.
References:
Korea Research Institute of Chemical Technology KR101508214, 2015, B1 Location in patent:Paragraph 0168-0172
618446-36-9
13 suppliers
inquiry

479553-01-0
150 suppliers
$24.00/100mg

124-38-9
134 suppliers
$175.00/23402
348640-06-2
337 suppliers
$6.00/250mg

479553-01-0
150 suppliers
$24.00/100mg
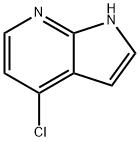
55052-28-3
477 suppliers
$6.00/1g

479553-01-0
150 suppliers
$24.00/100mg
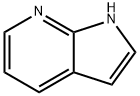
271-63-6
573 suppliers
$9.00/5g

479553-01-0
150 suppliers
$24.00/100mg