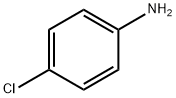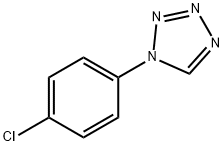
1H-TETRAZOLE, 1-(4-CHLOROPHENYL)- synthesis
- Product Name:1H-TETRAZOLE, 1-(4-CHLOROPHENYL)-
- CAS Number:25108-32-1
- Molecular formula:C7H5ClN4
- Molecular Weight:180.59
Yield:25108-32-1 98%
Reaction Conditions:
with Caswell No. 744A;1,1',1''?(1,3,5?triazine?2,4,6?triyl)tris(3?methyl?1H?imidazol?3?ium) iodide at 60; for 1 h;Microwave irradiation;Green chemistry;Wavelength;
Steps:
General procedure for microwave-assisted preparation of 5-substituted 1H-tetrazoles from aldehyde
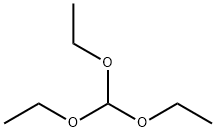
General procedure: A round bottom flask was charged with aldehyde (1.0 mmol), hydroxyl amine hydrochloride (1.5 mmol), NaN3(1.5 mmol) and TAIm[I] (2.5 mL). The resulting mixture was irradiated under microwave at 150 W and 60 °C for appropriate time that was obtained by continuous TLC monitoring at various times interval. After completion of the reaction, hot H2O (3.0 mL) and hot EtOAc (3.0 mL) was added to the mixture, and the product was further extracted three times to EtOAc. The resulting combined organic layers were dried over MgSO4. The desired 5-substituted 1H-tetrazole product was purified by recrystallization in EtOH. For the preparation of 5-substituted 1H-tetrazoles from nitrile, aryl nitrile (1.0 mmol) was used as a precursor under MW 300 W at 80 °C. A same procedure was applied for the microwave-assisted preparation of 1-substituted 1H-1,2,3,4-tetrazoles by arylamine (1.0 mmol) and triethyl orthoformate(1.2 mmol). Also, a same procedure was served for TAIm[I] recycling from the reaction mixture.
References:
Jasim, Saade Abdalkareem;Tanjung, Faisal Amri;Sharma, Sandhir;Mahmoud, Mustafa Z.;Kadhim, Sally B.;Kazemnejadi, Milad [Research on Chemical Intermediates,2022,vol. 48,# 8,p. 3547 - 3566]
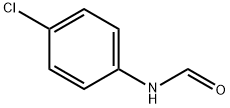
2617-79-0
52 suppliers
$60.00/100mg

25108-32-1
12 suppliers
inquiry
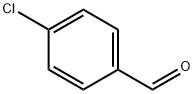
104-88-1
645 suppliers
$5.00/25g

25108-32-1
12 suppliers
inquiry
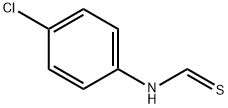
26060-33-3
0 suppliers
inquiry

25108-32-1
12 suppliers
inquiry