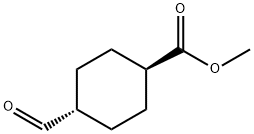
(1r,4r)-methyl 4-formylcyclohexanecarboxylate synthesis
- Product Name:(1r,4r)-methyl 4-formylcyclohexanecarboxylate
- CAS Number:54274-80-5
- Molecular formula:C9H14O3
- Molecular Weight:170.21
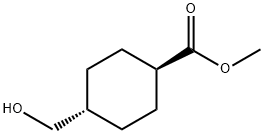
110928-44-4

54274-80-5
The general procedure for the synthesis of trans-methyl-4-(hydroxymethyl)cyclohexanecarboxylic acid from methyl trans-4-(hydroxymethyl)cyclohexanecarboxylate was as follows: oxalyl chloride (7.74 g, 61.0 mmol) was slowly added to an anhydrous dichloromethane (400 mL) solution of dimethyl sulfoxide (9.53 g, 122 mmol) at -78 °C. After removing the cooling bath, the reaction mixture was stirred at -50 °C for 5 min. Subsequently, a solution of methyl trans-4-hydroxymethyl-cyclohexanecarboxylate (8.75 g, 50.8 mmol) in dichloromethane (108 mL) was added at -65 °C. After continued stirring for 30 minutes, triethylamine (25.7 g, 254 mmol) was added. Fifteen minutes after the addition was complete, the cooling bath was removed. The reaction mixture was quenched with 1 M aqueous hydrochloric acid (152 mL, 152 mmol) at -10 °C. The organic and aqueous layers were separated, and the organic layer was washed sequentially with two parts of 250 mL of water and one part of 100 mL of brine, and then dried with anhydrous sodium sulfate. Finally, a yellow oily trans-methyl-4-formylcyclohexanecarboxylic acid (9.3 g, quantitative yield) was obtained by vacuum concentration, which could be used directly in the next reaction without further purification.MS m/e: 170 (M+).

110928-44-4
112 suppliers
$19.00/100mg

54274-80-5
101 suppliers
$30.00/1g
Yield:54274-80-5 100%
Reaction Conditions:
Stage #1:trans-(4-hydroxymethyl)cyclohexane-1-carboxylic acid methyl ester with oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -65; for 0.5 h;
Stage #2: with triethylamine in dichloromethane at -65; for 0.25 h;
Steps:
trans-4-Formyl-cyclohexanecarboxylic acid methyl ester
trans-4-Formyl-cyclohexanecarboxylic acid methyl ester
To a solution of dimethylsulfoxide (9.53 g, 122 mmol) in dry dichloromethane (400 ml) was slowly added oxalyl chloride (7.74 g, 61.0 mmol) at -78° C.
The cooling bath was removed and the reaction mixture was stirred at -50° C. for 5 min.
A solution of trans-4-hydroxymethyl-cyclohexanecarboxylic acid methyl ester (8.75 g, 50.8 mmol) in dichloromethane (108 ml) was added at -65° C.
Stirring for 30 minutes was followed by addition of triethylamine (25.7 g, 254 mmol).
The cooling bath was removed 15 minutes after completed addition.
The reaction mixture was quenched with 1 M aqueous hydrochloric acid solution (152 ml, 152 mmol) at -10° C.
The layers were separated.
The organic layer was washed with two 250 ml-portions of water and one 100-ml portion of brine, dried over anhydrous sodium sulfate and concentrated in vacuo to give the title compound as yellow oil (9.3 g, quantitative), which was used in the next step without further purification. MS m/e: 170 (M+)
References:
HOFFMANN-LA ROCHE INC.;Dolente, Cosimo;Schnider, Patrick US2014/221350, 2014, A1 Location in patent:Paragraph 0168
891828-63-0
0 suppliers
inquiry

54274-80-5
101 suppliers
$30.00/1g
1571-08-0
344 suppliers
$10.00/5g

54274-80-5
101 suppliers
$30.00/1g

135655-75-3
18 suppliers
$52.00/100mg

54274-80-5
101 suppliers
$30.00/1g
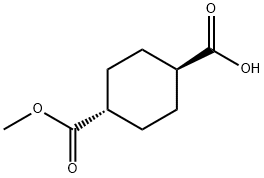
15177-67-0
219 suppliers
$6.00/1g

54274-80-5
101 suppliers
$30.00/1g