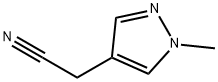
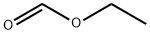2-(1-methyl-1H-pyrazol-4-yl)-3-oxopropanenitrile synthesis
- Product Name:2-(1-methyl-1H-pyrazol-4-yl)-3-oxopropanenitrile
- CAS Number:1039364-93-6
- Molecular formula:C7H7N3O
- Molecular Weight:149.15
Yield:1039364-93-6 95%
Reaction Conditions:
with potassium tert-butylate in 1,2-dimethoxyethane at 85; for 29 h;
Steps:
5
The compound of formula Xl (10.49 g, 86.68 mmol) and ethyl formate (15.15 ml_, 187.5 mmol, 2.16 equiv.) were dissolved in anhydrous DME (25 ml_) and added drop-wise to a suspension of potassium- terf-butoxide (17.01 g of 95%, 144.3 mmol, 1.66 equiv.) in anhydrous DME (90 ml_) in an open pressure tube. After addition was complete, the tube was sealed and stirred at 85 0C for 29 hours. After cooling, the resulting thick suspension was diluted with water (400 ml_) to yield a solution of pH 8, and was extracted with ethyl acetate (3 x 400 ml_). The aqueous solution was then acidified to pH 4 with concentrated hydrochloric acid (11 ml_) resulting in the formation of a white precipitate. This suspension was then filtered, and the recovered solid was washed with water and dried under vacuum to yield the compound of formula XII as an off-white solid (11.66 g, 90% yield). Extraction of the aqueous filtrate with ethyl acetate (2 x 500 ml_), followed by washing with brine, drying with sodium sulfate, filtering and concentrating resulted in an additional quantity of the product (0.74 g, 5% yield).
References:
WO2008/153870,2008,A1 Location in patent:Page/Page column 31
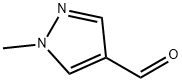
25016-11-9
246 suppliers
$5.00/1g

1039364-93-6
7 suppliers
inquiry