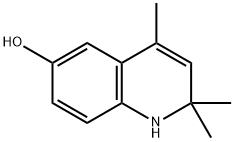
2,2,4-TRIMETHYL-1,2-DIHYDRO-QUINOLIN-6-OL synthesis
- Product Name:2,2,4-TRIMETHYL-1,2-DIHYDRO-QUINOLIN-6-OL
- CAS Number:72107-05-2
- Molecular formula:C12H15NO
- Molecular Weight:189.25
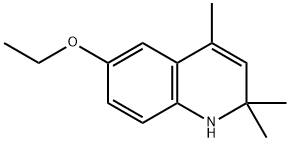
91-53-2
463 suppliers
$15.00/10g

7732-18-5
507 suppliers
$12.69/100ml

72107-05-2
31 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
Stage #1:Ethoxyquin with boron tribromide in dichloromethane at -78 - 20; for 2 h;
Stage #2:water at 0;
Steps:
7 Example 7: Synthesis of 4-Allylsulfanylmethyl-2, 2-dimethyl-6-thiophen-3-yl-1, 2- dihydroquinoline
Example 7: Synthesis of 4-Allylsulfanylmethyl-2, 2-dimethyl-6-thiophen-3-yl-1, 2- dihydroquinoline S Suzuki /I -S/I zu-l 7 A solution of 31 g of 6-ethoxy-2,2, [4-TRIMETHYL-1,] 2-dihydroquinoline in 300 mL of dichloromethane was treated with 30 [ML] of boron tribromide dropwise at-78°C. After the addition of boron tribromide, the cooling bath was removed and the reaction mixture was allowed to warm to room temperature. The resulting mixture was stirred at room temperature for 2 hours. The reaction mixture was then poured into crushed ice and the pH was adjusted to 9 with sodium bicarbonate. The product was the extracted into EtOAc. The organic solution was washed with water and brine, dried over magnesium sulfate, filtered, and concentrated in vacuo. The residue was purified by filtering through a short plug of silica gel eluting with 25% EtOAc in hexanes solution to give 12.4 g of 2, 2, 4-trimethyl-1, 2-dihydroquinolin-6-ol as a light brown solid. A solution of 12.4 g of 2, 2, [4-TRIMETHYL-1,] 2-dihydroquinilin-6-ol and 20 mL of triethyl amine in 360 mL of dichloromethane was treated with 12.1 mL [OF TRIFLUOROMETHANESULFONIC] anhydride, added dropwise at [0°C.] The resulting mixture was allowed to warm to room temperature and stirred at that temperature for 1 hour. The reaction mixture was quenched with saturated sodium bicarbonate solution and the product was extracted into ethyl ether. The ethyl ether layer was washed with water and brine, dried over magnesium sulfate, filtered, and concentrated in vacuo. The residue was purified by flash chromatography on silica gel (eluting with 20% EtOAc in hexanes) to give 19.3 g of trifluoromethanesulfonic acid 2,2, 4-trimethyl- [1,] 2-dihydroquinolin-6-yl ester as a light brown solid. To a dry 50 mL round bottom flask charged with a mixture of [TRIFLUOROMETHANESULFONIC] acid 2,2, [4-TRIMETHYL-1,] 2-dihydroquinolin-6-yl ester (0.50 g, 1.56 mmol), Pd [(OAC)] 2 (0.035 g, 0.156 mmol), [DI-TERT-BUTYLBIPHENYLPHOSPHINE] (DTBBPP) (0.093 g, 0.312 mmol), and KF (0.27 g, 4.67 mmol) suspended in THE (5 mL), was added 3-thiopheneboronic acid (0.30 g, 2.33 mmol). The flask was evacuated and flushed three times with argon and the reaction mixture was allowed to stir at room temperature for 2 days. The reaction mixture was filtered through diatomaceous earth and diluted with EtOAc (50 mL). The mixture was sequentially washed with water (10 mL), [NAOH] (1.0 M, 10 mL), and brine (10 mL). The organic phase was dried over sodium sulfate [(NA2S04),] filtered, and concentrated in vacuo. The crude residue was purified by chromatography (silica gel, 30% dichloromethane in hexanes) to give 2,2, 4- [TRIMETHYL-6-THIOPHEN-3-YL-1,] 2-dihydroquinoline (0.128 g, 32%). 2,2, [4-TRIMETHYL-6-THIOPHEN-3-YL-1,] 2-dihydroquinoline was brominated with NBS using the procedure described in Example 1 and then coupled with allyl mercaptan using the procedure described in Example 2 to provide 22 mg of the title compound as a oil.
References:
BOEHRINGER INGELHEIM PHARMACEUTICALS, INC. WO2004/18429, 2004, A2 Location in patent:Page 79

91-53-2
463 suppliers
$15.00/10g

72107-05-2
31 suppliers
$45.00/50mg
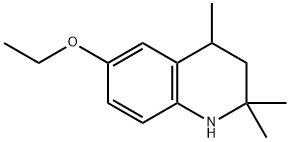
16489-90-0
18 suppliers
$144.00/25mg

72107-05-2
31 suppliers
$45.00/50mg