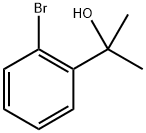
2-(2-Bromophenyl)-2-propanol synthesis
- Product Name:2-(2-Bromophenyl)-2-propanol
- CAS Number:7073-69-0
- Molecular formula:C9H11BrO
- Molecular Weight:215.09
917-64-6
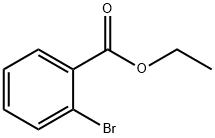
6091-64-1

7073-69-0
To a solution of 26.50 g (0.14 mol) of ethyl 2-bromobenzoate (4a) dissolved in 35 mL of anhydrous diethyl ether at -5°C was slowly added methylmagnesium iodide Grignard's reagent prepared from 9.1 g (0.37 mol) of magnesium and 53.3 g (0.37 mol) of freshly distilled methyl iodide in 85 mL of anhydrous ether. Upon completion of the reaction, the reaction was quenched by the addition of 38% ammonium chloride solution (58 mL). The ether layer was separated by decantation and the remaining pasty precipitate was extracted with ether (3 x 35 mL). All ether layer extracts were combined and dried over anhydrous sodium sulfate, followed by evaporation of the solvent. The product 2-(2-bromophenyl)-2-propanol 19.40 g was obtained in 82% yield and 95% purity (GLC assay). Physical properties of the product: boiling point 99-100 °C (5 mmHg), d420=1.1576, nD20=1.5420; MRD measured value 46.43, calculated value 46.55. Comparison of literature data: melting point 23.7 °C, boiling point 79.2 °C (2.2 mmHg), nD20=1.5416. IR spectra characteristic absorption peaks (ν, cm-1) : 3460, 1175 (OH), 1370, 1385 [C(CH3)2], 690 (C-Cl).1H NMR spectral data (δ, ppm): 1.64s (6H, CH3), 2.46s (1H, OH), 6.9-7.7m (4H, Harom). Elemental analysis measured values (%): C 63.00, H 6.42, Cl 20.71; calculated values (%) for molecular formula C9H11ClO: C 63.35, H 6.50, Cl 20.78.
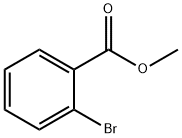
610-94-6
313 suppliers
$13.00/25g
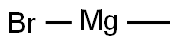
75-16-1
275 suppliers
$12.00/10ml

7073-69-0
86 suppliers
$22.00/250mg
Yield: 98.2%
Reaction Conditions:
Stage #1:2-bromobenzoic acid methyl ester;methylmagnesium bromide in tetrahydrofuran at 20;Cooling with ice;
Stage #2: with hydrogenchloride in water; pH=5 - 6
Steps:
2 Example 2: Preparation of compound II (taking methyl 2-bromobenzoate as an example)
Methyl 2-bromobenzoate (226g, 1.05mol) was dissolved in THF (1.6L), and 3MCH3MgBr (1.05L, 3.15mol) was added dropwise under ice-water bath conditions. After the addition was completed, a white syrupy liquid was formed. Slowly rise to room temperature and stir until the reaction is complete. Under ice-water bath conditions, use HCl (4.5L, 0.5 M, 2.25mol) to quench the reaction slowly. After the addition is complete, continue to stir for 0.5h, then add 2N HCl (0.5 L, 1.00mol) to make the pH value 5-6 . Add MTBE (1L), separate the organic phase, extract the aqueous phase with MTBE (2×0.5L), combine the organic phases, wash with sodium bicarbonate solution (2×0.3L), dry with anhydrous sodium sulfate, filter, and concentrate , 222 g (98.2% yield) of compound II was obtained as a solid.
References:
CN112430177, 2021, A Location in patent:Paragraph 0067; 0068
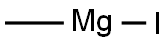
917-64-6
151 suppliers
$45.00/10ml

6091-64-1
177 suppliers
$10.00/1g

7073-69-0
86 suppliers
$22.00/250mg
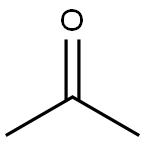
67-64-1
0 suppliers
$17.30/10ml
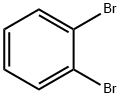
583-53-9
322 suppliers
$10.00/5g

7073-69-0
86 suppliers
$22.00/250mg

6091-64-1
177 suppliers
$10.00/1g
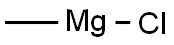
676-58-4
292 suppliers
$15.00/25ml

7073-69-0
86 suppliers
$22.00/250mg

610-94-6
313 suppliers
$13.00/25g
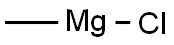
676-58-4
292 suppliers
$15.00/25ml

7073-69-0
86 suppliers
$22.00/250mg