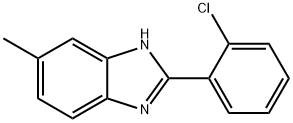
2-(2-CHLORO-PHENYL)-5-METHYL-1H-BENZOIMIDAZOLE synthesis
- Product Name:2-(2-CHLORO-PHENYL)-5-METHYL-1H-BENZOIMIDAZOLE
- CAS Number:14225-76-4
- Molecular formula:C14H11ClN2
- Molecular Weight:242.7
Yield:14225-76-4 82%
Reaction Conditions:
with methanesulfonic acid;silica gel in neat (no solvent) at 90; for 6 h;Green chemistry;
Steps:
General Procedure
General procedure: A mixture of 4-Chlorobenzaldehyde (420 mg, 3 mmol, 2) and ortho-phenylenediamine (346 mg, 3.2 mmol, 1) were mixed with methanesulphonic acid(1 mL) and silica (0.5 g) in a 50 mL round-bottomed flask and it was heated in an oil bath at 900C. Time to time the reaction was observed by silica coated TLC plate in presence of ethyl acetate and petroleum ether as an eluent. The reaction completed after the formation of brown spot within 4-9 h in iodine chamber. Then the crude product was cooled and diluted with ethylacetate, and filtered. The filtrate was washed three times with 50 mL saturated aqueous bicarbonate solution. It was washed three times with 30 mL brine solution. Then it was concentrated in a rotary evaporator under vacuum at room temperature. The product was crystallized directly from hot aqueous ethanol to produce the pure products. For bis benzimidazole same method was used but change of temperature was 1200C. Character analysis of the product was compared by FT-IR, 1H NMR and 13C NMR data with a good quality of known compounds.
References:
Datta, Arup;Roy, Sanjay [Oriental Journal of Chemistry,2020,vol. 36,# 3,p. 537 - 543]
326903-05-3
2 suppliers
inquiry

14225-76-4
12 suppliers
inquiry
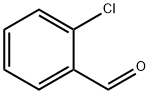
89-98-5
668 suppliers
$15.00/25g
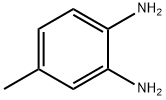
496-72-0
328 suppliers
$9.00/1g

14225-76-4
12 suppliers
inquiry
![1H-Benzimidazole, 2-(2-chlorophenyl)-1-[(2-chlorophenyl)methyl]-6-methyl-](/CAS/20210305/GIF/110877-18-4.gif)
110877-18-4
0 suppliers
inquiry