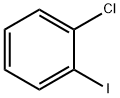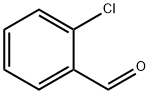
2-Chlorobenzaldehyde synthesis
- Product Name:2-Chlorobenzaldehyde
- CAS Number:89-98-5
- Molecular formula:C7H5ClO
- Molecular Weight:140.57
2-Chlorobenzaldehyde can also be produced by oxidation of 2-chlorobenzyl chloride with N-oxides of tertiary amines or with dilute nitric acid.
https://pubchem.ncbi.nlm.nih.gov/compound/2-Chlorobenzaldehyde
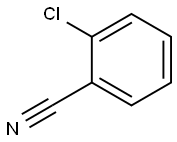
873-32-5
571 suppliers
$9.00/1g

89-98-5
671 suppliers
$15.00/25g
Yield:89-98-5 96 %Chromat.
Reaction Conditions:
with lithium diisobutylmorpholinoaluminum hydride in tetrahydrofuran;hexane at 0; for 0.5 h;
Steps:
Partial Reduction of Tertiary Amides and AromaticNitriles to Aldehydes with LDBMOA.
General procedure: The following procedure for reduction of N,N-dimethylbenzamide with LDBMOA is representative. To a solution of N,N-dimethylbenzamide (0.149 g, 1.0 mmol) in THF (10.0 mL) containing naphthalene as an internal standard was added LDBMOA (2.4 mL, 0.5 M in THF-hexanes, 1.2 mmol) at 0 °C. After 30 min, the reaction mixture was hydrolyzed with 1 N aq HCl (10 mL) and extracted with diethyl ether. The combined organic layers were dried over anhydrous magnesium sulfate, and filtered. After the removal of solvents in vacuum and purification of residue by column chromatography on silica gel gave benzaldehyde (0.103 g, 97%).
References:
Kim, Yu Ri;An, Duk Keun [Bulletin of the Korean Chemical Society,2012,vol. 33,# 12,p. 4194 - 4196]

13086-95-8
0 suppliers
inquiry

89-98-5
671 suppliers
$15.00/25g

24133-53-7
2 suppliers
$28.60/1g

89-98-5
671 suppliers
$15.00/25g
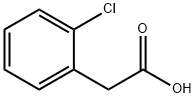
2444-36-2
452 suppliers
$5.00/25g

89-98-5
671 suppliers
$15.00/25g