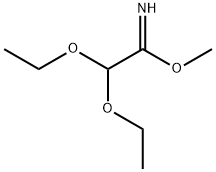
2,2-diethoxy-ethanimidic acid methyl ester synthesis
- Product Name:2,2-diethoxy-ethanimidic acid methyl ester
- CAS Number:76742-48-8
- Molecular formula:C7H15NO3
- Molecular Weight:161.2
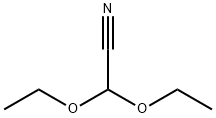
6136-93-2

124-41-4

76742-48-8
The general procedure for the synthesis of 2,2-diethoxy-1-methoxyethanimidamide from diethoxyacetonitrile and sodium methanol was as follows: first, diethoxyethanimidamide hydrochloride was synthesized according to the literature method. This was done as follows: to a solution of methanol (8.4 mL) containing sodium methanolate (0.09 g, 1.6 mmol) was added diethoxyacetonitrile (2.00 g, 15.5 mmol), and the reaction mixture was stirred at 0 °C for 4 h, and then returned to room temperature. Solid carbon dioxide (0.5 g) was added, followed by evaporation of most of the methanol. Ether was added to the residue and filtered to remove the resulting sodium carbonate. Concentration of the filtrate gave methyl diethoxyacetimidate as a colorless oil (2.47 g, 99% yield), which could be used in the next reaction without further purification. The resulting oil was dissolved in methanol (4 mL), ammonium chloride (0.82 g, 15.3 mmol) was added and the resulting mixture was stirred at room temperature overnight. Upon completion of the reaction, the mixture was filtered and the methanol was removed under vacuum. The residual oily substance was cooled to -78 °C, allowed to solidify and subsequently washed with ether (3 mL). The solid product was collected by filtration, recovered to room temperature and dried under vacuum to give the final diethoxyacetamidine hydrochloride (1.64 g, 58% overall yield in two steps) as a white solid. Its 1H NMR (400 MHz, DMSO-d6) data were as follows: 9.15 (br, 4H), 5.29 (s, 1H), 3.61 (q, 4H, J = 6.8 Hz), 1.18 (t, 6H, J = 6.8 Hz).

6136-93-2
148 suppliers
$10.00/1g

76742-48-8
44 suppliers
inquiry
Yield: 92%
Reaction Conditions:
with carbon dioxide;sodium methylate in methanol;diethyl ether
Steps:
236.a Preparation of Compound 236 in Table 7
a) To a solution of sodium methoxide (3.54 ml of a 25% wt. solution in methanol, 15.5 mmol) in methanol (80 ml) was added diethoxyacetonitrile (215 ml, 155 mmol) and the reaction mixture stirred for 4 hours at room temperature. Solid carbon dioxide was added and most of the methanol was removed in vacuo. Diethyl ether (30 ml) was added and the sodium carbonate removed by filtration. The residue was concentrated to afford methyl diethoxyacetimidate as a colourless oil (22 g, 92% yield) which was used with no further purification. MS (+ve ESI): 162 (M+H)+.
References:
AstraZeneca AB US7235559, 2007, B1

67-56-1
790 suppliers
$7.29/5ml-f

6136-93-2
148 suppliers
$10.00/1g

124-41-4
730 suppliers
$12.00/25g

76742-48-8
44 suppliers
inquiry