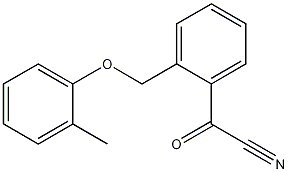
2-(2-methylphenoxymethyl)benzoyl cyanide synthesis
- Product Name:2-(2-methylphenoxymethyl)benzoyl cyanide
- CAS Number:143211-11-4
- Molecular formula:C16H13O2N
- Molecular Weight:251.2799
Yield:143211-11-4 96.2%
Reaction Conditions:
in water;toluene at 55; under 3750.38 Torr; for 0.00555556 h;Flow reactor;Industrial scale;Temperature;
Steps:
1-3
(1) Take 1.3 kg of 2-(2-methylphenoxy)-methylenebenzoyl chloride at room temperature,Toluene is added, and a 25% toluene solution of 2-(2-methylphenoxy)-methylenebenzoyl chloride is prepared.Then, 269 g of sodium cyanide was taken and added to purified water to prepare a 20% sodium cyanide aqueous solution.(2) Set the temperature of the reaction module to 55°C;(3) Place the toluene solution of 2-(2-methylphenoxy)methylene benzoyl chloride in channel A,Put the sodium cyanide aqueous solution into channel B, turn on the metering pumps of channel A and channel B,Set the flow rate of channel A to 27ml/min, and set the pump pressure to 0.5MPa; set the flow rate of channel B to 6.5ml/min,The pump pressure is 0.5MPa (the molar ratio of 2-(2-methylphenoxy)-methylene benzoyl chloride to sodium cyanide is 1:1.05 at this flow rate);The reaction module is set to 4,At the same time, the solution is pumped into the reaction module of the microchannel reactor for reaction,The total time that the reaction liquid stays in the reaction module is 20s;(4) The reaction liquid flowing out of the reaction module of the microchannel reactor enters the water after being cooled by the ice-water bath of the cooling coil,Water washing and liquid separation, take the toluene phase;(5) Dissolve the toluene phase to obtain 1.22 kg of 2-(2-methylphenoxymethyl)benzoyl nitrile,The yield was 96.2%.
References:
CN112279782,2021,A Location in patent:Paragraph 0007; 0030-0062
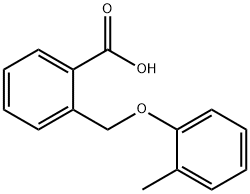
108475-90-7
16 suppliers
inquiry

143211-11-4
2 suppliers
inquiry
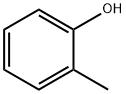
95-48-7
461 suppliers
$19.00/25g

143211-11-4
2 suppliers
inquiry
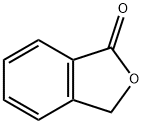
87-41-2
489 suppliers
$6.00/10g

143211-11-4
2 suppliers
inquiry
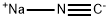
![Benzoyl chloride, 2-[(2-methylphenoxy)methyl]-](/CAS/20200331/GIF/143211-21-6.gif)