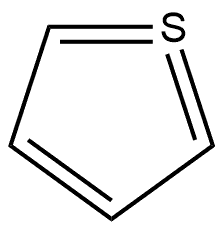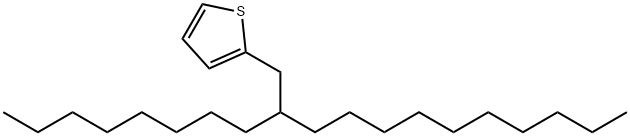
2-(2-octyldodecyl) synthesis
- Product Name:2-(2-octyldodecyl)
- CAS Number:929895-63-6
- Molecular formula:C24H44S
- Molecular Weight:364.67
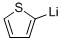
2786-07-4
18 suppliers
$50.00/25ml
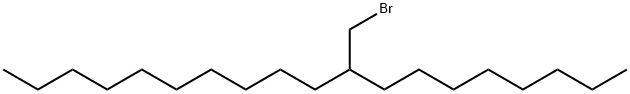
69620-20-8
81 suppliers
$33.00/25g

929895-63-6
14 suppliers
inquiry
Yield:929895-63-6 64%
Reaction Conditions:
in tetrahydrofuran;hexane at -78;Inert atmosphere;Reflux;
Steps:
Synthesis of 2-(2-octyldodecyl)thiophene (4)
To 1 M 2-thienyllitium solution (100 mL, 100 mmol) in THF/hexane was slowly added 2-octyl-1-bromododecane (1) (11.8 g, 32.8 mmol) at -78 °C under an argon atmosphere, and the solution was refluxed overnight. After quenching the reaction with 5 wt% NH4Cl aq., the product was extracted with hexane. The hexane solution was washed with brine,and dried over MgSO4. After the filtration, the filtrate was condensed under the reduced pressure. The crude productwas then purified by distillation at 147 °C/0.055 Torr to afford 4 as pale yellow oil (7.68 g, 64%). 1H NMR(300 MHz,CDCl3, δ, ppm, 25 °C): 7.11 (dd, J=5.1, 1.5 Hz, 1H, aromatic proton), 6.91 (dd, J=5.3, 3.3 Hz, 1H, aromatic proton),6.75 (dd, J=3.3, 1.2 Hz, 1H, aromatic proton), 2.75 (dd, J=6.8 , 0.6 Hz, 2H, thiophene-CH2-), 1.67-1.56 (m, 1H,thiophene-CH2-CH), 1.34-1.19 (m, 32H, -CH2-), 0.88 (t, J=6.6 Hz, 6H, -CH3). 13C NMR (75 MHz, CDCl3, δ,ppm, 25 °C): 144.50, 126.66, 125.10, 123.04, 40.17, 34.39, 33.32-26.74 (14 carbons), 22.85 (2 carbons), 14.29 (2carbons). IR (NaCl), v (cm-1): 2924 (alkyl C-H).
References:
Aoyagi, Koutarou;Shoji, Yu;Otsubo, Saika;Kawauchi, Susumu;Ueda, Mitsuru;Matsumoto, Hidetoshi;Higashihara, Tomoya [Bulletin of the Chemical Society of Japan,2014,vol. 87,# 10,p. 1083 - 1093] Location in patent:supporting information
5333-42-6
283 suppliers
$30.00/10g

929895-63-6
14 suppliers
inquiry

69620-20-8
81 suppliers
$33.00/25g

929895-63-6
14 suppliers
inquiry
1003-09-4
484 suppliers
$10.00/25g

69620-20-8
81 suppliers
$33.00/25g

929895-63-6
14 suppliers
inquiry