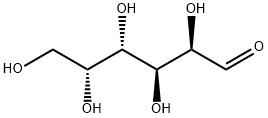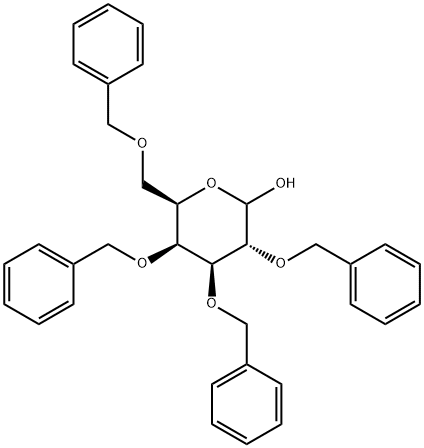
2,3,4,6-TETRA-O-BENZYL-D-GALACTOPYRANOSE synthesis
- Product Name:2,3,4,6-TETRA-O-BENZYL-D-GALACTOPYRANOSE
- CAS Number:6386-24-9
- Molecular formula:C34H36O6
- Molecular Weight:540.65
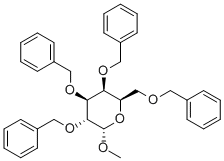
53008-63-2
87 suppliers
$50.00/5G

6386-24-9
63 suppliers
$96.31/1G
Yield:6386-24-9 87%
Reaction Conditions:
with hydrogenchloride;strontium (III) chloride hexahydrate in water;acetic acid at 70;
Steps:
B. Synthesis of 2,3,4,6-Tetra-O-benzyl-D-glycopyranoses 3, 5 and 11.
General procedure: To a stirred solution of methyl 2,3,4,6-tetra-O-benzyl-α-D-glycopyranosides 3-2, 4 5-2 or 11-2 (4.99 g, 9 mmol) in 25 mL of glacial acetic acid containing SrCl2·6H2O (0.24 g, 0.9 mmol) heated at 70°C was added 4 mL of 5 M hydrochloric acid. The stirring was continued at 70°C until reaction completed as indicated by TLC analysis (typically within 2-3 h). The reaction mixture was poured into 300 mL of ice-water while stirring immediately after the reaction completed, and the resulting mixture was extracted with three 100-mL portions of dichloromethane. The combined extracts were washed successively with saturated sodium bicarbonate and brine, dried over anhydrous sodium sulfate and evaporated on a rotary evaporator to afford an oily residue, which was purified by column chromatography to yield the pure 2,3,4,6-tetra-O-benzyl-D-glycopyranoses 3, 5 and 11 as anomeric mixtures.
References:
Wei, Peng;Zhang, Datong;Gao, Zhigang;Cai, Wenqing;Xu, Weiren;Tang, Lida;Zhao, Guilong [Synthetic Communications,2015,vol. 45,# 12,p. 1457 - 1470] Location in patent:supporting information
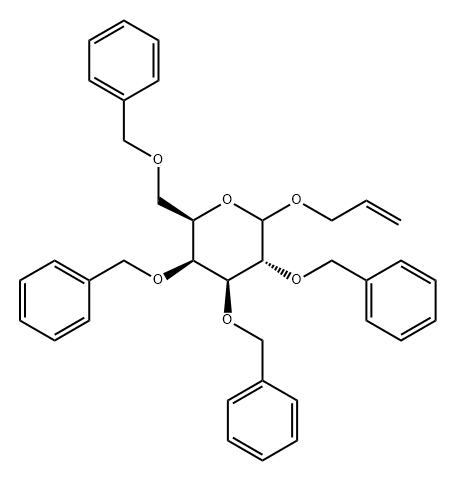
214490-83-2
0 suppliers
inquiry

6386-24-9
63 suppliers
$96.31/1G
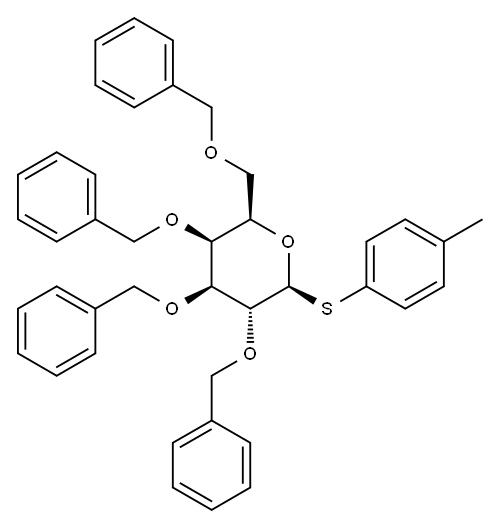
211678-08-9
10 suppliers
inquiry

6386-24-9
63 suppliers
$96.31/1G
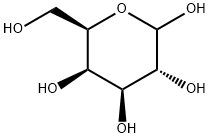
10257-28-0
76 suppliers
$138.00/100g

100-44-7
659 suppliers
$13.50/250G

6386-24-9
63 suppliers
$96.31/1G