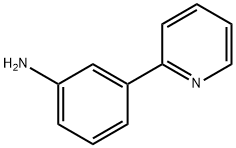
2-(3-AMINOPHENYL)PYRIDINE synthesis
- Product Name:2-(3-AMINOPHENYL)PYRIDINE
- CAS Number:15889-32-4
- Molecular formula:C11H10N2
- Molecular Weight:170.21
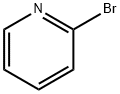
109-04-6
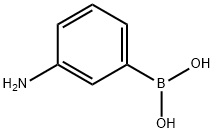
30418-59-8

15889-32-4
General procedure for the synthesis of 3-(2-pyridyl)aniline from 2-bromopyridine and 3-aminophenylboronic acid: cf. Example 31; to a suspension of 2-bromopyridine (0.5 g, 3.2 mmol), 3-aminophenylboronic acid (0.49 g, 3.2 mmol), anhydrous K2CO3 (0.87 g, 6.3 mmol) and Pd(PPh3)4 (0.36 g, 0.32 mmol) in a suspension of 1,2-dimethoxyethane (50 mL) and water (0.66 mL) under argon protection. The reaction mixture was heated at 80 °C under argon atmosphere overnight. After completion of the reaction, it was cooled to room temperature and extracted by adding water and EtOAc. The organic and aqueous phases were separated, and the combined organic phase was dried with Na2SO4 and concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography using a mixed solvent of gradient polarity hexane-EtOAc as eluent to give 0.22 g of 3-(2-pyridinyl)aniline (Yield: 42%). LC-MS (Method 1): tR = 1.46 min; m/z = 171.2 [M + H]+.
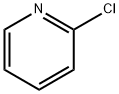
109-09-1
0 suppliers
$10.60/1gm:
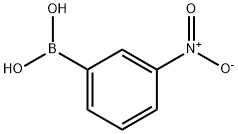
13331-27-6
354 suppliers
$5.00/1g

15889-32-4
74 suppliers
$65.00/100mg
Yield: 78%
Reaction Conditions:
with potassium carbonate;hydrazine hydrate in water;N,N-dimethyl-formamide at 80; for 9 h;Suzuki-Miyaura Coupling;
Steps:
2.4 Preparation of Aminobiphenyls in the Presenceof Ni7-Pd3(at)BIHPS
General procedure: Aryl halide (1.0 mmol), phenylboronic acid (1.1 mmol),K2CO3(2.0 mmol), Ni7-Pd3BIHPS (0.03 g) and H2O/DMF (2:1, 3 mL) were mixed and heated at 80 °C for anappropriate time (monitored by TLC). Then, hydrazinehydrate (80 wt%, 6 eq) was added to the reaction vessel.After completion of the reduction process, the mixturewas filtered and washed with water. The organic layer wasextracted with ethyl acetate (3 × 15 mL) and dried overMgSO4.Then the organic solution was concentrated andpurified by column chromatography to obtain the finalproduct.
References:
Beigbaghlou, Somayyeh Sarvi;Javad Kalbasi, Roozbeh;Marjani, Katayoun;Habibi, Azizollah [Catalysis Letters,2018,vol. 148,# 8,p. 2446 - 2458]

109-04-6
528 suppliers
$6.00/25g

30418-59-8
262 suppliers
$6.00/1g

15889-32-4
74 suppliers
$65.00/100mg
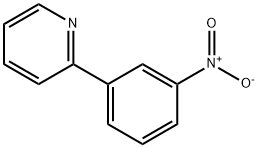
4253-79-6
10 suppliers
inquiry

15889-32-4
74 suppliers
$65.00/100mg
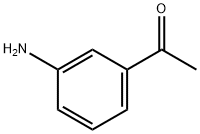
99-03-6
402 suppliers
$6.00/10g

109-76-2
310 suppliers
$10.00/5mL

15889-32-4
74 suppliers
$65.00/100mg
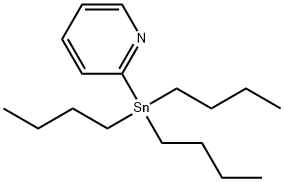
17997-47-6
177 suppliers
$18.00/1g
591-19-5
334 suppliers
$10.00/10g

15889-32-4
74 suppliers
$65.00/100mg