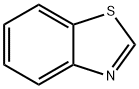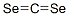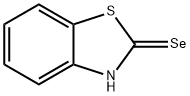
2(3H)-Benzothiazoleselone(9CI) synthesis
- Product Name:2(3H)-Benzothiazoleselone(9CI)
- CAS Number:10200-72-3
- Molecular formula:C7H5NSSe
- Molecular Weight:214.15
Yield:10200-72-3 60 %
Reaction Conditions:
with selenium;lithium tert-butoxide in N,N-dimethyl-formamide at 80;Sealed tube;
Steps:
1
Add benzothiazole (0.20mmol), selenium powder (0.40mmol), and lithium tert-butoxide (0.40mmol) to the sealed tube, dissolve it with 2mL N,N dimethylformamide (DMF) in a nitrogen atmosphere, and the reaction tube After sealing, place in an 80°C oil bath and stir for 12 hours.After the reaction was monitored by TLC, the reaction mixture was cooled to room temperature, extracted with ethyl acetate, the combined organic phases were dried over anhydrous Na2SO4, and the solvent was evaporated under reduced pressure.The residue was purified by column chromatography (eluted with a mixed solvent of petroleum ether and ethyl acetate) to obtain a yellow solid product with a yield of 60.0%.
References:
CN115650926,2023,A Location in patent:Paragraph 0074; 0084-0086
615-20-3
249 suppliers
$19.00/25g

10200-72-3
3 suppliers
inquiry