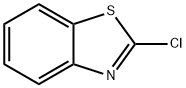
2-Chlorobenzothiazole synthesis
- Product Name:2-Chlorobenzothiazole
- CAS Number:615-20-3
- Molecular formula:C7H4ClNS
- Molecular Weight:169.63
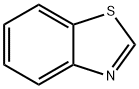
95-16-9

615-20-3
The general procedure for the synthesis of 2-chlorobenzothiazole from benzothiazole was as follows: benzothiazole (1 mmol, 135.9 mg) and carbon tetrachloride (1.1 mmol, 169.2 mg) were placed in a 10 mL round bottom flask. 5 mL of N,N-dimethylformamide and sodium tert-butoxide (4.0 mmol, 384.4 mg) were added to the reaction system and stirred thoroughly to ensure that the reactants were homogeneously mixed. The reaction was carried out at room temperature for 3 h, during which the progress of the reaction was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction mixture was poured into water and extracted with dichloromethane. The organic phase was separated and dried with anhydrous sodium sulfate. Subsequently, the dichloromethane was removed by rotary evaporator to obtain the crude product. The crude product was purified by silica gel column chromatography using a mixed solvent of petroleum ether and ethyl acetate as eluent (30:1, v/v). 2-Chlorobenzothiazole (brown oily liquid, 149.2 mg) was finally obtained in 88% yield.

95-16-9
421 suppliers
$14.14/10gm:

615-20-3
252 suppliers
$19.00/25g
Yield:615-20-3 95.3%
Reaction Conditions:
with chlorine;trichlorophosphate in chlorobenzene at 80 - 100;Reagent/catalyst;Temperature;Solvent;
Steps:
1-5 Example 4:
With a cooler and a hydrogen chloride tail gas absorption device,Can be stirred and equipped with a chlorine gas introduction tubeIn a 2000 ml reaction flask,600 g of benzothiazole, 500 g of chlorobenzene and 10 g of phosphorus oxychloride catalyst were introduced, and the temperature was raised to 80 ° C.Start to pass chlorine gas at (3 ~ 5) g / min, the reaction is maintained at (80 ~ 100) ° C. Insulation reaction until the HPLC detection of the benzothiazole content in the system is less than 0.5%, the chlorine is stopped, the amount of chlorine is about 300 grams . After the reaction, the nitrogen gas is purged of excess chlorine, which is a 2-chlorobenzothiazole chlorobenzene solution.After distillation under reduced pressure, chlorobenzene and a small amount of raw materials are removed for the next batch.The remaining material was subjected to a high vacuum of 0.099 mPa, and distilled to 165 ° C to obtain 2-chlorobenzothiazole, 725 g, content 99%.The yield was 95.3%.
References:
CN109456282,2019,A Location in patent:Paragraph 0017-0024
149-30-4
683 suppliers
$5.00/25g

615-20-3
252 suppliers
$19.00/25g
136-95-8
412 suppliers
$7.00/25g

615-20-3
252 suppliers
$19.00/25g
615-21-4
229 suppliers
$45.00/10mg

615-20-3
252 suppliers
$19.00/25g
147-93-3
467 suppliers
$7.00/5g

615-20-3
252 suppliers
$19.00/25g