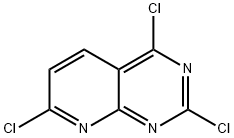
2,4,7-Trichloropyrido[2,3-d]pyrimidine synthesis
- Product Name:2,4,7-Trichloropyrido[2,3-d]pyrimidine
- CAS Number:938443-20-0
- Molecular formula:C7H2Cl3N3
- Molecular Weight:234.47
![7-Chloropyrido[2,3-d]pyrimidine-2,4-diol](/CAS/GIF/938443-19-7.gif)
938443-19-7
63 suppliers
inquiry
![2,4,7-Trichloropyrido[2,3-d]pyrimidine](/CAS/GIF/938443-20-0.gif)
938443-20-0
110 suppliers
$57.00/100mg
Yield: 48%
Reaction Conditions:
Stage #1:7-chloropyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione with N-ethyl-N,N-diisopropylamine in toluene at 70; for 0.5 h;
Stage #2: with trichlorophosphate in toluene at 20 - 100; for 2.5 h;
Steps:
d
d) 2,4, 7-Trichloro-pyrido[2,3-d]pyrimidine (Inter.5); To a stirred 0.5 M suspension of the dione (Inter. 4)(1 equiv.) in anhydrous toluene under an inert atmosphere was slowly added diisopropylethylamine (3 equiv.). The reaction mixture was then heated to 700C for 30 minutes and then cooled to room temperature prior to the addition of POCl3 (3 equivalents). The reaction was then heated to 100°C for 2.5 hours before being cooled and concentrated in vacuo to give a crude slurry which was then suspended in EtOAc and filtered through a thin pad of Celite. The filtrate was concentrated in vacuo to give a brown, oil which was dissolved in CH2Cl2 and stirred over silica gel for 30 minutes. After this time the silica was removed by filtration, the filtrate concentrated and the crude residue purified by flash chromatography (SiO2) to give the title compound in analytically pure form (48% yield, 96% purity), m/z (LC-MS, ESP): 234 [M+H]+ R/T = 4.21 mins
References:
Location in patent:Page/Page column 57; 59
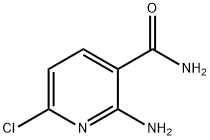
64321-24-0
79 suppliers
$45.00/10mg
![2,4,7-Trichloropyrido[2,3-d]pyrimidine](/CAS/GIF/938443-20-0.gif)
938443-20-0
110 suppliers
$57.00/100mg
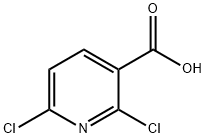
38496-18-3
361 suppliers
$6.00/1g
![2,4,7-Trichloropyrido[2,3-d]pyrimidine](/CAS/GIF/938443-20-0.gif)
938443-20-0
110 suppliers
$57.00/100mg
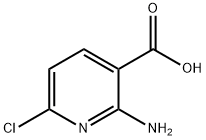
58584-92-2
139 suppliers
$18.00/250mg
![2,4,7-Trichloropyrido[2,3-d]pyrimidine](/CAS/GIF/938443-20-0.gif)
938443-20-0
110 suppliers
$57.00/100mg
![Pyrido[2,3-d]pyrimidine-2,4,7(1H,3H,8H)-trione](/CAS/20200331/GIF/258282-54-1.gif)
258282-54-1
2 suppliers
inquiry
![2,4,7-Trichloropyrido[2,3-d]pyrimidine](/CAS/GIF/938443-20-0.gif)
938443-20-0
110 suppliers
$57.00/100mg