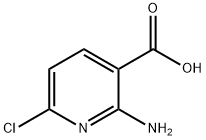
2-Amino-5-chloropyridine-3-carboxylic acid synthesis
- Product Name:2-Amino-5-chloropyridine-3-carboxylic acid
- CAS Number:58584-92-2
- Molecular formula:C6H5ClN2O2
- Molecular Weight:172.57
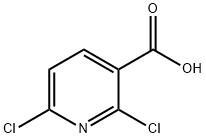
38496-18-3

58584-92-2
The general procedure for the synthesis of 2-amino-6-chloronicotinic acid (Inter.2) using 2,6-dichloronicotinic acid (Inter.I) as starting material is as follows: 1. 2,6-dichloronicotinic acid (Inter.I) (1 equiv.) was mixed with liquid ammonia to prepare a 0.6 M ammonia solution. 2. The suspension is transferred to a pressure vessel, sealed and slowly heated to 130°C. At this temperature, the system pressure reaches 18 bar. 3. The temperature and pressure conditions were maintained for 16 hours. 4. upon completion of the reaction, cool the mixture to room temperature. 5. carefully open the pressure vessel and pour the reaction mixture into ice-cold water (in the same volume as the volume of reactants). 6. Acidify the solution to pH 1-2 using concentrated hydrochloric acid, at which point a precipitate is formed. 7. Warm the acidic mixture to room temperature and continue stirring for 30 minutes. 8. Extract the suspension with ether (400 ml each time, 3 times). 9. The organic extracts were combined, filtered and the filtrate was concentrated under vacuum to give a white solid. 10. The resulting solid was dried over P2O5 to afford the target compound 2-amino-6-chloronicotinic acid (Inter.2) in 90% yield and 96% purity. The product can be used directly without further purification. Mass spectral data: m/z (LC-MS ESP): 173 [M + H]+, retention time R/T = 3.63 min.

38496-18-3
384 suppliers
$6.00/1g

58584-92-2
144 suppliers
$18.00/250mg
Yield:58584-92-2 93%
Reaction Conditions:
with ammonium hydroxide at 130; for 12 h;Sealed tube;
Steps:
68 2-Amino-6-chloropyridine-3-carboxylic acid
A solution of 2,6-dichloropyridine-3-carboxylic acid (3 g, 0.0157 mol) in aqueous ammonia (30 mL) was heated in a sealed tube at 130°C for 12 h. The reaction mixture was concentrated and the resulting crude solid was triturated with diethyl ether to afford the title compound (2.5 g, 93%). δΗ (400 MHz, DMSO-de) 8.02 (d, J 8.0 Hz, IH), 7.55 (s, 2H), 6.62 (d, J 8.0 Hz, IH). LCMS: mlz 173 (97.70%)
References:
UCB BIOPHARMA SPRL;BRACE, Gareth Neil;CHOVATIA, Prafulkumar Tulshibhai;FOULKES, Gregory;JOHNSON, James Andrew;JONES, Severine Danielle;KROEPLIEN, Boris;LECOMTE, Fabien Claude;LOKE, Pui Leng;LOWE, Martin Alexander;MANDAL, Ajay;NORMAN, Timothy John;PALMER, Christopher Francis;PÉREZ-FUERTES, Yolanda;PORTER, John Robert;SMYTH, Donald;TRANI, Giancarlo;UDDIN, Muhammed;ZHU, Zhaoning WO2016/198400, 2016, A1 Location in patent:Page/Page column 65
60-35-5
530 suppliers
$10.80/1g

38496-18-3
384 suppliers
$6.00/1g

58584-92-2
144 suppliers
$18.00/250mg
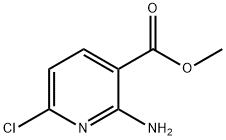
849805-25-0
75 suppliers
$28.00/100mg

58584-92-2
144 suppliers
$18.00/250mg

64-17-5
825 suppliers
$10.00/50g
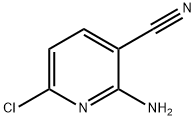
52471-07-5
66 suppliers
inquiry

1384985-48-1
0 suppliers
inquiry

58584-92-2
144 suppliers
$18.00/250mg

52471-07-5
66 suppliers
inquiry

58584-92-2
144 suppliers
$18.00/250mg