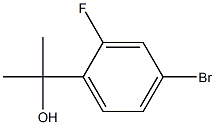
2-(4-bromo-2-fluorophenyl)propan-2-ol synthesis
- Product Name:2-(4-bromo-2-fluorophenyl)propan-2-ol
- CAS Number:749928-52-7
- Molecular formula:C9H10BrFO
- Molecular Weight:233.08

75-16-1
274 suppliers
$12.00/10ml
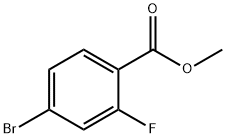
179232-29-2
236 suppliers
$9.00/5g

749928-52-7
13 suppliers
inquiry
Yield:749928-52-7 95%
Reaction Conditions:
in diethyl ether at 0 - 20; for 3 h;
Steps:
A.215.1
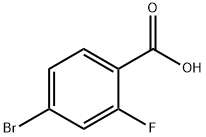
A solution of 4-bromo-2-fluorobenzoic acid (10 g) in anhydrous MeOH (200 mL) was added CONC. sulfuric acid (0.5 mL). The mixture was heated to reflux for 15 hours before it was cooled down to room temperature. The solvent was removed and the residue was taken up in EtOAc (100 mL) and washed with sat. NAHC03, brine and dried over NA2SO4. The crude product was taken into next step without further purification. To a solution of methyl 4-bromo-2-fluorobenzoate (12 g, 51.5 mmol) in anhydrous ether (140 mL) at 0 C was added MeMgBr (3.0 M, 70 g) dropwise. The mixture was slowly warmed up to room temperature and stirred for 3 hours. The reaction was quenched by the addition of saturated NH4CI and extracted with EtOAc (3 x 150 mL). The combined organic extracts were washed with brine, dried with NA2SO4 and evaporated to dryness. The mixture was purified by flash column chromatography (0-20 % EtOAc in hexanes) to give the product (12 g, 95% yield). 'H NMR (300 MHz, CDCI3) S : 1.62 (s, 6 H), 7.18-7. 23 (m, 1 H), 7.25-7. 28 (m, 1 H), 7.44-7. 49 (m, 1 H).
References:
WO2004/74270,2004,A2 Location in patent:Page 220-221
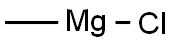
676-58-4
292 suppliers
$15.00/25ml
112704-79-7
534 suppliers
$15.00/5G

749928-52-7
13 suppliers
inquiry
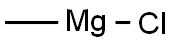
676-58-4
292 suppliers
$15.00/25ml

179232-29-2
236 suppliers
$9.00/5g

749928-52-7
13 suppliers
inquiry

67-56-1
790 suppliers
$9.00/25ml

112704-79-7
534 suppliers
$15.00/5G

749928-52-7
13 suppliers
inquiry