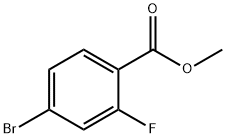
Methyl 4-bromo-2-fluorobenzoate synthesis
- Product Name:Methyl 4-bromo-2-fluorobenzoate
- CAS Number:179232-29-2
- Molecular formula:C8H6BrFO2
- Molecular Weight:233.03

67-56-1
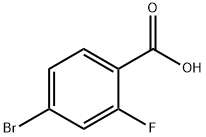
112704-79-7

179232-29-2
Step 1: To a stirred solution of 4-bromo-2-fluorobenzoic acid (15.0 g, 68.49 mmol, 1 eq.) in methanol (150 mL) was slowly added thionyl chloride (23.09 mL, 136.9 mmol, 2 eq.) at 0 °C. The reaction mixture was kept at 0 °C for 15 minutes before being brought to room temperature and stirring was continued for 12 hours. After completion of the reaction, methanol was removed by evaporation under reduced pressure and the residue was diluted with ethyl acetate (250 mL). The organic layer was washed sequentially with saturated aqueous sodium bicarbonate solution, brine (150 mL) and deionized water (150 mL). The ethyl acetate layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to give methyl 4-bromo-2-fluorobenzoate (15 g, 93% yield) as an off-white solid (LC-MS purity 99%; TLC conditions: ethyl acetate/petroleum ether (3:7), Rf value 0.8).

112704-79-7
534 suppliers
$15.00/5 g

18107-18-1
211 suppliers
$33.00/5g

179232-29-2
236 suppliers
$9.00/5g
Yield:179232-29-2 100%
Reaction Conditions:
in tetrahydrofuran;methanol;hexanes at 0 - 20; for 1 h;
Steps:
45.1
STEP 1 : To a solution of 4-bromo-2-fluorobenzoic acid (2.0 g, 9.1 mmol) in THF (24 mL) and methanol (6 mL) at 0 °C was added a solution of (trimethylsilyl)diazomethane in hexanes (2.0 M, 5.46 mL, 10.9 mmol). The yellow solution was allowed to gradually warm to room temperature over 1 h. The volatile materials were removed and the residue was treated with aqueous hydrochloric acid (1 M). The aqueous mixture was extracted with ethyl acetate. The organic extract was dried over magnesium sulfate, filtered, and concentrated to provide crude methyl 4- bromo-2-fluorobenzoate (2.7 g, quantitative yield). This material was used in the subsequent step without further purification. 1H NMR (400 MHz, CDCl3): 7.85-7.81 (m, IH), 7.38-7.33 (m, 2H), 3.93 (s, 3H); MS (EI) for C8H6BrFO2: 232, 234 (MH+).
References:
EXELIXIS, INC. WO2009/55077, 2009, A1 Location in patent:Page/Page column 447

67-56-1
790 suppliers
$7.29/5ml-f

112704-79-7
534 suppliers
$15.00/5 g

179232-29-2
236 suppliers
$9.00/5g

112704-79-7
534 suppliers
$15.00/5 g

74-88-4
357 suppliers
$15.00/10g

179232-29-2
236 suppliers
$9.00/5g

112704-79-7
534 suppliers
$15.00/5 g

179232-29-2
236 suppliers
$9.00/5g

67-56-1
790 suppliers
$7.29/5ml-f
151982-51-3
157 suppliers
$9.00/250mg

179232-29-2
236 suppliers
$9.00/5g