
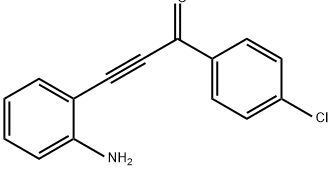
2-(4-CHLORO-PHENYLETHYNYL)-PHENYLAMINE synthesis
- Product Name:2-(4-CHLORO-PHENYLETHYNYL)-PHENYLAMINE
- CAS Number:221910-19-6
- Molecular formula:C14H10ClN
- Molecular Weight:227.69
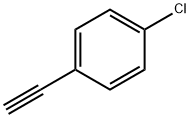
873-73-4
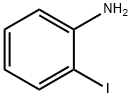
615-43-0

221910-19-6
PdCl2(PPh3)2 (88 mg, 0.125 mmol), CuI (24 mg, 0.125 mmol) and THF (5 mL) were added to an oven-dried, two-necked round-bottomed flask under standard reaction conditions. To this suspension, 2-iodoaniline (5.47 mg, 2.5 mmol) and triethylamine (702 μL, 5.0 mmol) were sequentially added. The reaction mixture was degassed by argon bubbling for 15 min. Subsequently, 4-chlorophenylacetylene (300 μL, 2.75 mmol) was added and the reaction mixture was stirred at room temperature. The progress of the reaction was monitored by thin-layer chromatography (TLC), and after complete consumption of 2-iodoaniline (about 2 h), the reaction mixture was filtered through diatomaceous earth and the solvent was removed by rotary evaporation to obtain the crude product. The crude product was further purified by column chromatography on silica gel (60-120 mesh) using ethyl acetate/hexane (1:9, v/v) as eluent, resulting in purified 2-((4-chlorophenyl)alkynyl)-aniline (400 mg, 83% yield).

873-73-4
229 suppliers
$5.00/100mg

615-43-0
426 suppliers
$5.00/5g

221910-19-6
25 suppliers
$17.00/100mg
Yield:221910-19-6 98%
Reaction Conditions:
with copper(l) iodide;tetrakis(triphenylphosphine) palladium(0);triethylamine in tetrahydrofuran at 20; for 72 h;Inert atmosphere;Schlenk technique;Sonogashira Cross-Coupling;
References:
Tussing, Sebastian;Ohland, Miriam;Wicker, Garrit;Fl?rke, Ulrich;Paradies, Jan [Dalton Transactions,2017,vol. 46,# 5,p. 1539 - 1545] Location in patent:supporting information
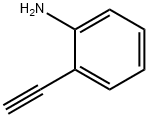
52670-38-9
146 suppliers
$33.00/250MG
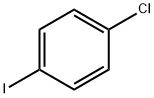
637-87-6
303 suppliers
$38.00/10g

221910-19-6
25 suppliers
$17.00/100mg

52670-38-9
146 suppliers
$33.00/250MG

637-87-6
303 suppliers
$38.00/10g
201230-82-2
1 suppliers
inquiry
108-91-8
631 suppliers
$10.00/5mL
57707-20-7
25 suppliers
$15.00/5mg
31661-58-2
15 suppliers
inquiry

221910-19-6
25 suppliers
$17.00/100mg

637-87-6
303 suppliers
$38.00/10g
201230-82-2
1 suppliers
inquiry
103529-16-4
39 suppliers
$57.50/1g

221910-19-6
25 suppliers
$17.00/100mg
221910-02-7
0 suppliers
inquiry

615-43-0
426 suppliers
$5.00/5g

221910-19-6
25 suppliers
$17.00/100mg