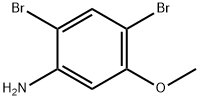
2,4-dibromo-5-methoxyaniline synthesis
- Product Name:2,4-dibromo-5-methoxyaniline
- CAS Number:35736-52-8
- Molecular formula:C7H7Br2NO
- Molecular Weight:280.94
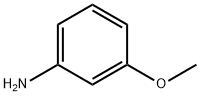
536-90-3
59557-92-5
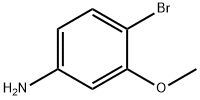
19056-40-7

35736-52-8
Using m-aminoanisole (5.00 g, 40.6 mmol) as starting material, it was dissolved in chloroform (40 mL) and the solution was cooled to 5 °C. N-bromosuccinimide (7.23 g, 40.6 mmol) was added to the cooled solution in batches over 1 hour. After addition, the reaction mixture was continued to be stirred in an ice bath at 5-10 °C for 4 hours. Subsequently, the mixture was warmed to room temperature and stirred overnight. Upon completion of the reaction, the reaction mixture was washed sequentially with 2M sodium hydroxide solution (50 mL) and water (60 mL) and then dried over anhydrous sodium sulfate. The solvent was removed by evaporation to give a dark brown viscous liquid. The crude product was separated by silica gel column chromatography (eluent: dichloromethane/hexane, 2:1), first eluting a mixture of 2-bromo-5-methoxyaniline and 2,4-dibromo-5-methoxyaniline. Subsequently, 4-bromo-3-methoxyaniline (860 mg, 10% yield) was eluted as peach-colored crystals with a melting point of 97-98 °C (literature value 93-94 °C); 1H NMR (400 MHz, CDCl3) δ 3.70 (broad single peak, 2H, NH2), 3.80 (single peak, 3H, OCH3), 6.16 (double peaks, J=2.5,8.4 Hz , 1H, ArH), 6.23 (double peaks, J=2.5 Hz, 1H, ArH), 7.23 (double peaks, J=8.4 Hz, 1H, ArH), and the data are in agreement with those reported in the literature. The eluate of the first major band was evaporated to dryness and re-separated by silica gel column chromatography (eluent: ethyl acetate/hexane, 1:2) to afford 2-bromo-5-methoxyaniline (830 mg, 10% yield) as an orange liquid; 1H NMR (400 MHz, CDCl3) δ 3.72 (single peak, 3H, OCH3), 4.07 (broad single peak, 2H NH2), 6.21 (double peak, J=2.9,8.8Hz, 1H, ArH), 6.30 (double peak, J=2.9Hz, 1H, ArH), 7.25 (double peak, J=8.8Hz, 1H, ArH), the data were in agreement with the literature reports. Subsequent elution of the second band gave 2,4-dibromo-5-methoxyaniline (2.66 g, 23% yield) as a brown liquid; Rf (80% ethyl acetate/dichloromethane) 0.87; IR (pure) νmax 3420, 3295, 3176, 2965, 2934, 1620, 1582, 1503, 1278, 1207, 1018 , 805 cm-1; 1H NMR (400 MHz, CDCl3) δ 3.80 (single peak, 3H, OCH3), 4.10 (broad single peak, 2H, NH2), 6.31 (single peak, 1H, ArH), 7.49 (single peak, 1H, ArH); 13C NMR (100 MHz, CDCl3) δ 56.1, 99.3, 99.3 , 99.7, 135.1, 144.3, 155.8; Elemental analysis calculated values (C7H7Br2NO): C 29.93, H 2.51, N 4.99; Measured values: C 30.29, H 2.24, N 4.95%.

536-90-3
298 suppliers
$10.00/5g

35736-52-8
44 suppliers
$17.00/250mg
Yield:35736-52-8 90%
Reaction Conditions:
with bromine in tetrachloromethane at 20;Cooling with ice;
References:
Bagmanov [Russian Journal of Applied Chemistry,2009,vol. 82,# 9,p. 1570 - 1576] Location in patent:experimental part

536-90-3
298 suppliers
$10.00/5g

59557-92-5
182 suppliers
$6.00/1g

19056-40-7
224 suppliers
$10.00/1g

35736-52-8
44 suppliers
$17.00/250mg